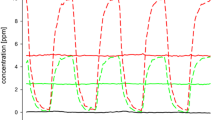
Abstract
Inhaled irritants can cause respiratory depression by simulating trigeminal nerves in the nasal cavity. This decrease in inhalation rate results in a decrease in the rate of the irritant gases flowing to the stimulated nerves, creating a complex feedback response. Previously, a model was created to describe how the presence of formaldehyde affects respiration in the rat. This ordinary differential equation model incorporated a model of the physiology of the upper respiratory tract of the rat and a model of the neurological control of the respiration rate due to signaling from the stimulated nerves in the nasal cavity. However, an optimal fit to data was not fully established. In the current study, the fit of the previously established model is reevaluated while incorporating the recovery of the ventilation rate after the end of exposure. Additionally, the dose-dependence of the adaptation time allowed by the previous model is more fully quantified, and the updated model predicts formaldehyde data well. Not only are the results of the previous study improved, the model is also shown to predict ventilation decrease in response to other irritants, specifically acrolein, ammonia, and sulfur dioxide. The model is expected to translate to predictions of other irritants with minor parameter changes.






Similar content being viewed by others
References
Alarie, Y. (1973). Sensory irritation by airborne chemicals. CRC Crit. Rev. Toxicol., 2, 299–363.
Alimohammadi, H., & Silver, W. L. (2000). Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem. Senses, 25, 61–66.
Andersen, M. E., & Sarangapani, R. (2001). Physiologically based clearance/extraction models for compounds metabolized in the nose: an example with methyl methacrylate. Inhal. Toxicol., 13, 397–414.
Barrow, C. S., Alarie, Y., Warrick, J. C., & Stock, M. F. (1977). Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health, 32, 68–76.
Casanova, M., Conolly, R., Everitt, J., Gross, B., Heck, H., Janszen, D., Kimbell, J., Lilly, P., Miller, F., Morgan, K., Preston, J., Schlosser, P., Subramaniam, R., Felter, S., & Overton, J. (1999). Formaldehyde: hazard characterization and dose-response assessment for carcinogenity by the route of inhalation, revised edn. Research Triangle Park: Chemical Industry Institute of Toxicology (CIIT)
Cassee, F. R., Arts, J. H. E., Groten, J. P., & Feron, V. J. (1996). Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Arch. Toxicol., 70, 329–337.
Chang, J. C. F., Steinhagen, W. H., & Barrow, C. S. (1981). Effect of single or repeated formaldehyde exposure on minute volume of B6C3F1 mice and F-344 rats. Toxicol. Appl. Pharmacol., 61, 451–459.
Conolly, R. B., Kimbell, J. S., Janszen, D., Schlosser, P. M., Kalisak, D., Preston, J., & Miller, F. J. (2003). Biologically motivated computational modeling of formaldehyde carcinogenity in the F344 Rat. Toxicol. Sci., 75, 432–447.
Dallas, C. E., Theiss, J. C., Harrist, R. B., & Fairchild, E. J. (1985). Effect of subchronic formaldehyde inhalation on minute volume and nasal deposition in Sprague-Dawley rats. J. Toxicol. Environ. Health, 16, 553–564.
Dutschmann, M., & Herbert, H. (1999). Pontine cholinergic mechanisms enhance trigeminally evoked respiratory suppression in the anesthetized rat. J. Appl. Physiol., 87(3), 1059–1065.
Frederick, C. B., Bush, M. L., Lomax, L. G., Black, K. A., Finch, L., Kimbell, J. S., Morgan, K. T., Subramaniam, R. P., Morris, J. B., & Ultman, J. S. (1998). Application of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry extrapolation of acidic vapors in the upper airways. Toxicol. Appl. Pharmacol., 152, 211–231.
Georgieva, A. V., Kimbell, J. S., & Schlosser, P. M. (2003). A distributed-parameter model for formaldehyde uptake and disposition in the rat nasal lining. Inhal. Toxicol., 15, 1435–1463.
Hext, P. M., & Bennett, I. P. (1993). Inhalation toxicology. In B. Ballantyne, T. Marrs, & P. Turner (Eds.), General and applied toxicology (Vol. 1, pp. 453–465). New York: Stockton Press.
Kimbell, J. S., Gross, E. A., Joyner, D. R., Godo, M. N., & Morgan, K. T. (1993). Application of computational fluid dynamics to regional dosimetry of inhaled chemicals in the upper respiratory tract of the rat. Toxicol. Appl. Pharmacol., 121(2), 253–263.
Kimbell, J. S., Gross, E. A., Richardson, R. B., Conolly, R. B., & Morgan, K. T. (1997). Correlation of regional formaldehyde flux predictions with the distribution of formaldehyde-induced squamous metaplasia in F344 rat nasal passages. Mutat. Res., 390, 143–154.
Kimbell, J. S., Overton, J. H., Subramaniam, R. P., Schlosser, P. M., Morgan, K. T., Conolly, R. B., & Miller, F. J. (2001a). Dosimetry modeling of inhaled formaldehyde: binning nasal flux predictions for quantitative risk assessment. Toxicol. Sci., 64, 111–121.
Kimbell, J. S., Subramaniam, R. P., Gross, E. A., Schlosser, P. M., & Morgan, K. T. (2001b). Dosimetry modeling of inhaled formaldehyde: comparisons of local flux predictions in the rat, monkey, and human nasal passages. Toxicol. Sci., 64, 100–110.
Kulle, T. J., & Cooper, G. P. (1975). Effects of formaldehyde and ozone on the trigeminal nasal sensory system. Arch. Environ. Health, 30, 237–243.
Mortola, J. P., Matsuoka, T., Saiki, C., & Naso, L. (1994). Metabolism and ventilation in hypoxic rats: effect of body mass. Respir. Physiol., 97, 225–234.
Nielsen, G. D., Hougaard, K. S., Larsen, S. T., Hammer, M., Wolkoff, P., Clausen, P. A., Wilkins, C. K., & Alarie, Y. (1999). Acute airway effects of formaldehyde and ozone in BALB/c mice. Hum. Exp. Toxicol., 18(6), 400–409.
Olufsen, M., Tran, H. T., & Ottesen, J. (2004). Modeling cerebral blood flow control during posture change from sitting to standing. Cardiovasc. Eng., Int. J., 4(1), 47–58.
Ottesen, J. T. (2000). Modelling the dynamical baroreflex-feedback control. Math. Comput. Model., 31(4–5), 167–173.
Strotmann, J., Wanner, I., Helfrich, T., Beck, A., & Breer, H. (1994). Rosto-caudal patterning of receptor-expressing olfactory neurones in the rat nasal cavity. Cell Tissue Res., 278, 11–20.
Viemari, J.-C., Bévengut, M., Coulon, P., & Hilaire, G. (2004). Nasal trigeminal inputs release the A5 inhibition received by the respiratory rhythm generator of the mouse neonate. J. Neurophysiol., 91, 746–758.
Wang, A. L., Blackford, T. L., & Lee, L.-Y. (1996). Vagal bronchopulmonary C-fibers and acute ventilatory response to inhaled irritants. Respir. Physiol., 104, 231–239.
Yeh, H. C., Schum, G. M., & Duggan, M. T. (1979). Anatomic models of the tracheobronchial and pulmonary regions of the rat. Anat. Rec., 195(3), 483–492.
Yokley, K. A., Tran, H., & Schlosser, P. M. (2008). Sensory irritation response in rats: modeling, analysis and validation. Bull. Math. Biol., 70, 555–588.
Acknowledgements
The author would like to thank the reviewers, Dr. Crista Arangala and Dr. Nicholas Luke for helpful comments in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: The Sensory Irritant Model Symbols
Compartments:
- NV:
-
The nasal vestibule compartment
- DR:
-
The respiratory compartment of the dorsal flow
- DO1:
-
The first olfactory compartment of the dorsal flow
- DO2:
-
The second olfactory compartment of the dorsal flow
- VR1:
-
The first respiratory compartment of the ventral flow
- VR2:
-
The second respiratory compartment of the ventral flow
- NP:
-
The nasopharynx compartment
- LRC:
-
The lower respiratory compartment
States:
- C i :
-
The concentration of formaldehyde in the air flow through compartment i (μmol/cm3)
- Q :
-
The ventilation rate, i.e., air flow rate through the respiratory tract (cm3/s)
Parameters:
- S i :
-
The surface area of compartment i (cm2)
- V i :
-
The volume of the air in compartment i (cm3)
- \(K_{\mathrm{gc},i}^{j}\) :
-
Mass transfer coefficient for compartment i for chemical j (cm/s)
- C inh :
-
The concentration of formaldehyde being inhaled (μmol/cm3)
- r(C i ):
-
Function representing the response due to chemical concentration that depends on occupancy chemical receptors on the nerve receptor surface (dimensionless)
- K D :
-
The receptor-chemical dissociation constant (μmol/cm3)
- r 0 :
-
The threshold parameter for r(C i ) (dimensionless)
- τ r :
-
An adaptation time constant for the firing rate n (s)
- k 0 :
-
The intrinsic sensitivity of the receptor to a chemical (dimensionless)
- w i :
-
A weighting factor for each compartment that will depend on nerve receptors (dimensionless)
- δ :
-
Small positive constant in order to achieve a nontrivial solution to the differential equation system (dimensionless)
- n :
-
Total firing rate relative to the maximum (dimensionless)
- Δn i :
-
Firing rate addition from receptors in the mucosa of compartment i (dimensionless)
- Q steady :
-
Sigmoidal function to represent the nerve change in response to formaldehyde as a function of n (cm3/h)
- τ Q :
-
Time constant characterizing the time it takes for the nervous response to take effect. The value of τ Q is denoted \(\tau_{Q_{1}}\) during the initial decrease in ventilation and is denoted \(\tau_{Q_{2}}\) during the recovery to steady state (s)
- Q min,Q max :
-
The maximum and minimum values of ventilation (cm3/s)
- p :
-
Parameter controlling the steepness of the sigmoid Q steady (dimensionless)
- α :
-
Positive constant in Q steady dependent on data (dimensionless)
- τ 0 :
-
Positive constant in the function for τ r (s/(μmol/cm3))
- c 1 :
-
Positive constant in the function for τ r (s)
- MW j :
-
Molecular weight of chemical j
Appendix B: The Sensory Irritant Model Equations
Equations (8)–(27) were used in the original model in Yokley et al. (2008). Equations (26) and (27) were used in a slightly modified way in the current study as was discussed in Sect. 3.2. Equation (28) was added in the current investigation.





















Appendix C: Parameter Values
The fixed parameters used in the model are contained in Tables 3 and 4. The choice of model parameters is more fully described in Yokley et al. (2008).
Rights and permissions
About this article
Cite this article
Yokley, K.A. Sensory Irritation Response in Rats II: Recovery and Dose-Dependence. Bull Math Biol 74, 1673–1690 (2012). https://doi.org/10.1007/s11538-012-9730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9730-4