Abstract
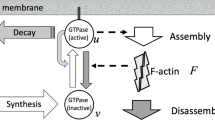
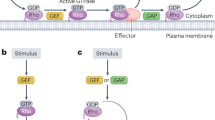
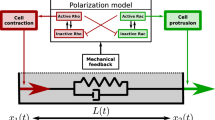
Cdc42, Rac, and Rho are small GTPases known to play a central role in signal transduction to the actin cytoskeleton. These proteins regulate cell motility, by affecting nucleation, uncapping, and depolymerization of actin filaments, and acto-myosin contractility. Studies of crosstalk and mutual feedbacks in these three proteins have led to a number of proposals for their interaction. At the same time, observations of the spatio-temporal dynamics of Rho-family proteins give evidence of spatial polarization and mutual exclusion between Cdc42/Rac and Rho. In this paper, we formulate a mathematical model to account for such observations, based on the known underlying biology of these proteins. We first investigate which of the crosstalk schemes proposed in the literature is consistent with observed dynamics, and then derive a simple model that can correctly describe these dynamics (assuming crosstalk is mediated via Rho GEFs). We show that cooperativity is an essential ingredient in the interactions of the proteins. The co-occurrence of a stable rest state with the possibility of fast spatial segregation can be related to bistability in a set of underlying ODEs in which the inactive forms of these proteins are fixed at a constant level. We show that the fast diffusion of the inactive forms is essential for stabilizing the transition fronts in the PDE formulation of the model, leading to robust spatial polarization, rather than traveling waves.
Similar content being viewed by others
References
Baird, D., Feng, Q., Cerione, R.A., 2005. The Cool-2/α-Pix protein mediates a Cdc42-Rac signaling cascade. Curr. Biol. 15, 1–10.
Benard, V., Bohl, B.P., Bokoch, G.M., 1999. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198–13204.
Benink, H.A., Bement, W.M., 2005. Concentric zones of active RhoA and Cdc42 around single cell wounds. J. Cell Biol. 168, 429–439.
Bishop, A.L., Hall, A., 2000. Rho GTPases and their effector proteins. Biochem. J. 348, 241–255.
Boukharov, A.A., Cohen, C.M., 1998. Guanine nucleotide-dependent translocation of RhoA from cytosol to high affinity membrane binding sites in human erythrocytes. Biochem. J. 330, 1391–1398.
Caron, E., 2003. Rac signalling: a radical view. Nat. Cell Biol. 5, 185–187.
Carr, J., Pego, R.L., 1989. Metastable patterns in solutions of u t =ε 2 u xx −f(u). Comm. Pure Appl. Math. 42, 523–576.
Cinquin, O., Demongeot, J., 2002. Positive and negative feedback: striking a balance between necessary antagonists. J. Theor. Biol. 216, 229–241.
Cross, M.C., Hohenberg, P.C., 1993. Pattern formation outside of equilibrium. Rev. Mod. Phys. 65, 851–1112.
Czuchra, A., Wu, X., Meyer, H., Van Hengel, J., Schroeder, T., Geffers, R., Rottner, K., Brakebusch, C., 2005. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol. Biol. Cell 16, 4473–4484.
Dawes, A.T., Edelstein-Keshet, L., 2007. Phosphoinositides and rho proteins spatially regulate actin polymerization to initiate and maintain directed movement in a one-dimensional model of a motile cell. Biophys. J. 92, 744–768.
DerMardirossian, C., Schnelzer, A., Bokoch, G.M., 2004. Phosphorylation of RhoGDI by Pak1 mediates dissociation of Rac GTPase. Mol. Cell 15, 117–127.
Edelstein-Keshet, L., 1988. Mathematical Models in Biology. Random House, New York.
Evers, E.E., Zondag, G.C.M., Malliri, A., Price, L.S., Ten Klooster, J.-P., Van Der Kammen, R.A., Collard, J.G., 2000. Rho family proteins in cell adhesion and cell migration. Eur. J. Cancer 36, 1269–1274.
Ferrell, J.E., Jr., 1996. Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends Biochem. Sci. 21, 460–466.
Ferrell, J.E., Jr., 2002. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148.
Gardner, T.S., Cantor, C.R., Collins, J.J., 2000. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342.
Giniger, E., 2002. How do Rho family GTPases direct axon growth and guidance? A proposal relating signaling pathways to growth cone mechanics. Differentiation 70, 385–396.
Gouzé, J.-L., 1998. Positive and negative circuits in dynamical systems. J. Biol. Syst. 6, 11–15.
Iijima, M., Huang, Y.E., Devreotes, P., 2002. Temporal and spatial regulation of chemotaxis. Dev. Cell 3, 469–478.
Iron, D., Ward, M.J., 2000. A metastable spike solution for a nonlocal reaction-diffusion model. SIAM J. Appl. Math. 60, 778–802.
Iron, D., Ward, M.J., 2001. Spike pinning for the Gierer-Meinhardt model. Math. Comput. Simul. 55, 419–431.
Itoh, R.E., Kurokawa, K., Ohba, Y., Yoshizaki, H., Mochizuki, N., Matsuda, M., 2002. Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22, 6582–6591.
Janetopoulos, C., Ma, L., Devreotes, P.N., Iglesias, P.A., 2004. Chemoattractant-induced phosphatidylinositol 3,4,5-trisphosphate accumulation is spatially amplified and adapts, independent of the actin cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 101, 8951–8956.
Jilkine, A., 2005. Modeling the interactions of small GTPases. Master’s thesis, University of British Columbia, Vancouver.
Kimura, K., Ito, M., Amano, M., Chihara, K., Fukata, Y., Nakafuku, M., Yamamori, B., Feng, J., Nakano, T., Okawa, K., Iwamatsu, A., Kaibuchi, K., 1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273, 245–248.
Kraynov, V.S., Chamberlain, C., Bokoch, G.M., Schwartz, M.A., Slabaugh, S., Hahn, K.M., 2000. Localized Rac activation dynamics visualized in living cells. Science 290, 333–337.
Krishnan, J., Iglesias, P.A., 2004. A modeling framework describing the enzyme regulation of membrane lipids underlying gradient perception in Dictyostelium cells. J. Theor. Biol. 229, 85–99.
Kurokawa, K., Itoh, R.E., Yoshizaki, H., Nakamura, Y.O.T., Matsuda, M., 2004. Coactivation of Rac1 and Cdc42 at lamellipodia and membrane ruffles induced by epidermal growth factor. Mol. Biol. Cell 15, 1003–1010.
Levchenko, A., Iglesias, P.A., 2002. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys. J. 82, 50–63.
Li, Z., Aizenman, C.D., Cline, H.T., 2002. Regulation of Rho GTPases by crosstalk and neuronal activity in vivo. Neuron 33, 741–750.
Li, Z., Dong, X., Wang, Z., Liu, W., Deng, N., Ding, Y., Tang, L., Hla, T., Zeng, R., Li, L., Wu, D., 2005. Regulation of PTEN by Rho small GTPases. Nat. Cell Biol. 7, 399–404.
Ma, L., Janetopoulos, C., Yang, L., Devreotes, P.N., Iglesias, P.A., 2004. Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in Dictyostelium cells. Biophys. J. 87, 3764–3774.
Mackay, D.J.G., Hall, A., 1998. Rho GTPases. J. Biol. Chem. 273, 20685–20688.
Marée, A.F.M., Jilkine, A., Dawes, A., Grieneisen, V.A., Edelstein-Keshet, L., 2006. Polarization and movement of keratocytes: a multiscale modeling approach. Bull. Math. Biol. 68, 1169–1211.
Matozaki, T., Nakanishi, H., Takai, Y., 2000. Small G-protein networks: their crosstalk and signal cascades. Cell. Signal. 12, 515–524.
Meili, R., Firtel, R.A., 2003. Two poles and a compass. Cell 114, 153–156.
Meinhardt, H., 1999. Orientation of chemotactic cells and growth cones: models and mechanisms. J. Cell Sci. 112, 2867–2874.
Michaelson, D., Silletti, J., Murphy, G., D’Eustachio, P., Rush, M., Philips, M.R., 2001. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152, 111–126.
Miki, H., Takenawa, T., 2003. Regulation of actin dynamics by WASP family proteins. J. Biochem. 134, 309–313.
Mitchison, T.J., Cramer, L.P., 1996. Actin-based cell motility and cell locomotion. Cell 84, 371–379.
Murray, J.D., 2002. Mathematical Biology I: An Introduction, 3rd edn. Springer, New York.
Nalbant, P., Hodgson, L., Kraynov, V., Toutchkine, A., Hahn, K.M., 2004. Activation of endogenous Cdc42 visualized in living cells. Science 305, 1615–1619.
Narang, A., 2006. Spontaneous polarization in eukaryotic gradient sensing: a mathematical model based on mutual inhibition of frontness and backness pathways. J. Theor. Biol. 240, 538–553.
Nobes, C.D., Hall, A., 1995. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62.
Olofsson, B., 1999. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell. Signal. 11, 545–554.
Parent, C.A., Devreotes, P.N., 1999. A cell’s sense of direction. Science 284, 765–770.
Pertz, O., Hodgson, L., Klemke, R.L., Hahn, K.M., 2006. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440, 1069–1072.
Pestonjamasp, K.N., Forster, C., Sun, C., Gardiner, E.M., Bohl, B., Weiner, O., Bokoch, G.M., Glogauer, M., 2006. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood 108, 2814–2820.
Postma, M., Van Haastert, P.J.M., 2001. A diffusion-translocation model for gradient sensing by chemotactic cells. Biophys. J. 81, 1314–1323.
Postma, M., Bosgraaf, L., Loovers, H.M., Van Haastert, P.J.M., 2004. Chemotaxis: signalling modules join hands at front and tail. EMBO Rep. 5, 35–40.
Raftopoulou, M., Hall, A., 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32.
Ridley, A.J., 2001a. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477.
Ridley, A.J., 2001b. Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722.
Ridley, A.J., Paterson, H.F., Johnston, C.L., Diekmann, D., Hall, A., 1992. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410.
Rohatgi, R., Ma, L., Miki, H., Lopez, M., Kirchhausen, T., Takenawa, T., Kirschner, M.W., 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97, 221–231.
Sako, Y., Hibino, K., Miyauchi, T., Miyamoto, Y., Ueda, M., Yanagida, T., 2000. Single-molecule imaging of signaling molecules in living cells. Single Mol. 1, 159–163.
Sakumura, Y., Tsukada, Y., Yamamoto, N., Ishii, S., 2005. A molecular model for axon guidance based on cross talk between rho GTPases. Biophys. J. 89, 812–822.
Sander, E.E., Ten Klooster, J.-P., Van Delft, S., Van Der Kammen, R.A., Collard, J.G., 1999. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009–1022.
Srinivasan, S., Wang, F., Glavas, S., Ott, A., Hofmann, F., Aktories, K., Kalman, D., Bourne, H.R., 2003. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160, 375–385.
Stanyon, C.A., Bernard, O., 1999. LIM-kinase1. Int. J. Biochem. Cell Biol. 31, 389–394.
Subramanian, K.K., Narang, A., 2004. A mechanistic model for eukaryotic gradient sensing: spontaneous and induced phosphoinositide polarization. J. Theor. Biol. 231, 49–67.
Sun, C.X., Downey, G.P., Zhu, F., Koh, A.L.Y., Thang, H., Glogauer, M., 2004. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood 104, 3758–3765.
Takai, Y., Sasaki, T., Matozaki, T., 2001. Small GTP-binding proteins. Physiol. Rev. 81, 153–208.
Thomas, R., 1981. On the relation between the logical structure of systems and their ability to generate multiple steady states or sustained oscillations. In: J. della Dora, J. Demongeot, B. Lacolle (Eds.), Numerical methods in the study of critical phenomena. Springer Series in Synergetics, vol. 9, pp. 180–193. Springer, Berlin.
Thomas, R., Kaufman, M., 2001. Multistationarity, the basis of cell differentiation and memory. I. Structural conditions of multistationarity and other nontrivial behavior. Chaos 11, 170–179.
Tolias, K.F., Hartwig, J.H., Ishihara, H., Shibasaki, Y., Cantley, L.C., Carpenter, C.L., 2000. Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol. 10, 153–156.
Turing, A.M., 1952. The chemical basis of morphogenesis. Phil. Trans. Roy. Soc. Lond. B 237, 37–72.
Van Keymeulen, A., Wong, K., Knight, Z.A., Govaerts, C., Hahn, K.M., Shokat, K.M., Bourne, H.R., 2006. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J. Cell Biol. 174, 437–445.
Van Leeuwen, F.N., Kain, H.E.T., Van Der Kammen, R.A., Michiels, F., Kranenburg, O.W., Collard, J.G., 1997. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 139, 797–807.
Verkhovsky, A.B., Svitkina, T.M., Borisy, G.G., 1999. Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11–20.
Vidali, L., Chen, F., Cicchetti, G., Ohta, Y., Kwiatkowski, D.J., 2006. Rac1-null mouse embryonic fibroblasts are motile and respond to platelet-derived growth factor. Mol. Biol. Cell 17, 2377–2390.
Wedlich-Soldner, R., Altschuler, S., Wu, L., Li, R., 2003. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231–1235.
Weiner, O.D., Neilsen, P.O., Prestwich, G.D., Kirschner, M.W., Cantley, L.C., Bourne, H.R., 2002. A PtdInsP3- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513.
Wheeler, A.P., Wells, C.M., Smith, S.D., Vega, F.M., Henderson, R.B., Tybulewicz, V.L., Ridley, A.J., 2006. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J. Cell Sci. 119, 2749–2757.
Wong, K., Pertz, O., Hahn, K., Bourne, H., 2006. Neutrophil polarization: spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc. Natl. Acad. Sci. U.S.A. 103, 3639–3644.
Xu, J., Wang, F., Van Keymeulen, A., Herzmark, P., Straight, A., Kelly, K., Takuwa, Y., Sugimoto, N., Mitchison, T., Bourne, H.R., 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell 114, 201–214.
Zerial, M., Huber, L.A. (Eds.) 1995. Guidebook to the Small GTPases. Oxford University Press, Oxford.
Zhang, B., Zheng, Y., 1998. Regulation of RhoA GTP hydrolysis by the GTPase-activating proteins p190, p50RhoGAP, Bcr, and 3BP-1. Biochemistry 37, 5249–5257.
Zheng, Y., 2001. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 26, 724–732.
Zondag, G.C.M., Evers, E.E., Ten Klooster, J.-P., Janssen, L., Van Der Kammen, R.A., Collard, J.G., 2000. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J. Cell Biol. 149, 775–782.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jilkine, A., Marée, A.F.M. & Edelstein-Keshet, L. Mathematical Model for Spatial Segregation of the Rho-Family GTPases Based on Inhibitory Crosstalk. Bull. Math. Biol. 69, 1943–1978 (2007). https://doi.org/10.1007/s11538-007-9200-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-007-9200-6