Abstract
Kristen Rat Sarcoma viral oncogene (KRAS) mutations are one of the most common oncogenic drivers found in 12–14% of non-small cell lung cancer (NSCLC) and 4% of colorectal cancer tumors. Although previously difficult to target, sotorasib and adagrasib are now approved for previously treated NSCLC patients with KRAS G12C mutations. In preclinical studies, divarasib was 5 to 20 times as potent and up to 50 times as selective as sotorasib and adagrasib. While sotorasib met its primary endpoint in the phase III second line study against docetaxel, the progression-free survival (PFS) benefit was small and no overall survival (OS) benefit was observed. Adagrasib has demonstrated clinical benefit in the phase I/II KRYSTAL-1 study setting, however, 44.8% of patients reported grade 3 or higher toxicities. Divarasib has been studied in a phase I dose expansion cohort with promising efficacy [objective response (ORR) 53.4% and PFS 13.1 months]. Although most patients reported toxicities, the majority were low-grade and manageable with supportive care. Here we discuss these results in the context of the evolving KRAS G12C landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sotorasib and adagrasib have been FDA approved for KRAS G12C NSCLC. |
Divarasib is a potent and selective KRAS G12C inhibitor. |
Early results show promising efficacy and safety of divarasib. |
Divarasib may have the potential to change the KRAS G12C treatment landscape. |
1 Introduction
Kristen Rat Sarcoma viral oncogene (KRAS) mutations are one of the most common oncogenic drivers found in 12–14% of non-small cell lung cancer (NSCLC) and 4% of colorectal cancer (CRC) tumors [1, 2]. In NSCLC, KRAS mutations are associated with younger age and smoking history. The KRAS G12C mutation consists of a glycine-to-cysteine mutation at position 12, which inhibits guanosine triphosphate (GTP) hydrolysis, keeping KRAS in the active state. Until recently, KRAS mutations had no targeted agents owing to smooth surface with lack of binding sites, small dimension, and high affinity for GTP/guanosine diphosphate (GDP) [3, 4]. In a meta-analysis and systematic review of 10,153 NSCLC patients, authors found that KRAS G12C-mutated tumors had worse overall survival (OS) with a hazard ratio (HR) of 1.42 [95% confidence interval (CI) 1.10–1.84] but similar disease-free survival (DFS) (HR 2.36, 95% CI 0.64–8.16) [5]. KRAS G12C-mutated cases had a similar OS to other KRAS mutations (HR 1.03, 95% CI 0.84–1.26) but a higher programmed death ligand 1 (PD-L1) expression [odds ratio (OR) 1.37, 95% CI 1.11–1.70].
Currently two KRAS G12C inhibitors are available for previously treated NSCLC patients: sotorasib and adagrasib. Recently, divarasib has shown similar efficacy and tolerability as prior KRAS G12C inhibitors. Here we discuss the phase I results of divarasib.
2 Preclinical Studies
2.1 Pharmacodynamics
Preclinical studies of GDC-6036 (divarasib) demonstrated a median half-maximal inhibitory concentration (IC50) in the sub-nanomolar range [6]. Divarasib was over 18,000-fold more selective for mutant G12C cell lines than wild type G12C. Divarasib also demonstrated greater potency and selectivity in vitro than other KRAS G12C inhibitors (sotorasib and adagrasib) as presented in Table 1. Divarasib resulted in complete tumor growth inhibition in multiple KRAS G12C positive cell lines and xenograft cell models. On the basis of these preclinical findings, human dose projections estimated that doses under 400 milligrams (mg) would result in 90% alkylation (IC90) in H2122.
2.2 Pharmacokinetics
In the phase I trial, pharmacokinetic data was evaluable in four patients with solid tumors. After a single 400 mg dose of divarasib, the average half-life was 17.6 h (+/− 2.7 h) [7]. Steady state was assessed at cycle 1 day 8 or cycle 2 day 1. In total, 76 patients were evaluated at steady state and demonstrated mean maximum concentration was 657 nanograms per milliliter (+/− 185) and mean area under the curve was 9130 nanograms times h per milliliter (+/− 3160).
3 Phase I Study Design
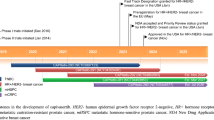
Results of a phase I trial of divarasib were recently published (NCT04449874) [7]. The study design is outlined in Fig. 1. Inclusion criteria included advanced or metastatic solid tumor, KRAS G12C mutation, progressed on at least one line of prior therapy, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and no active untreated brain metastases. Patients with treated brain metastases were included in the study population. A total of 137 participants were enrolled, including 60 with NSCLC, 55 with colorectal cancer, and 22 other solid tumors.
Divarasib was administered in 21-day cycles. The dose escalation included doses of 50 mg, 100 mg, 200 mg, and 400 mg daily. The primary outcomes included safety, pharmacokinetics, investigator-evaluated antitumor activity, and biomarkers of both response and resistance.
3.1 Efficacy
Within the NSCLC cohort, investigators reported an objective response rate (ORR) of 53.4% (95% CI 39.9–66.7) with a median progression-free survival (PFS) of 13.1 months (95% CI 8.8, not reached). Within the colorectal cancer subgroup objective response rate (ORR) was 29.1% (95% CI 17.6–42.9) with a median PFS of 5.6 months (95% CI 4.1–8.2). The number of median lines of therapy was 2 (range: 0–8). The investigators note that responses were also observed in other solid malignancies. Serial circulating tumor deoxyribonucleic acid (DNA) showed a decline in KRAS G12C allele frequency associated with clinical response.
3.2 Tolerability
Adverse events were reported in 127 patients (93%) taking divarasib. This included 11% grade 3 and 1% grade 4 adverse events. There were no grade 5 events reported. The most common adverse events in the NSCLC cohort include nausea (n = 27, 78%), vomiting (n = 38, 63%), diarrhea (n = 36, 60%), and fatigue (n = 16, 27%). Dose reductions occurred in 19 (14%) and the agent was discontinued in 4 participants (3%).
4 Discussion
Divarasib has demonstrated efficacy in KRAS G12C-mutated NSCLC with ORR 53.4% and PFS 13.1 months. Although adverse events were frequent (93%), the vast majority were grade 1 or 2. These positive phase I results have prompted further investigation with phase II/III as well as combination agent trials which are summarized in Table 2. In colorectal cancers divarasib is being studied in combination with cetuximab, FOLFOX, and FOLFIRI (NCT04929223). Early data on the divarasib plus cetuximab combination show an ORR of 62% [8]. Although grade 3 and higher adverse events were reported in 38% of patients, treatment discontinuation was not required in most cases. In NSCLC divarasib is being studied as monotherapy in phase II/III trials (NCT03178552) and in combination with pembrolizumab (NCT05789082). Additionally, an exploratory SHP2 inhibitor, GDC-1971, is being added to divarasib (NCT04449874).
Sotorasib (Lumakras, Amgen) was one of the first KRAS G12C inhibitors and was approved by the Federal Drug Administration (FDA) in 2021 in the second-line treatment of NSCLC with KRAS G12C mutations based on CodeBreak100. The trial enrolled 174 participants who received sotorasib at 960 mg daily after a median of two prior lines of therapy. In a pooled analysis of phase I/II sotorasib data, ORR was 41% (95% CI 33.3–48.4) with PFS of 6.3 months (95% CI 5.3–8.2) [9]. The duration of response (DOR) was 12.3 months (95% CI 7.1–15.0) with median OS of 12.5 months (95% CI 10.0–17.8). Importantly there were no adverse events that led to treatment discontinuation. In another analysis of real-world outcomes with sotorasib in recurrent, metastatic KRAS G12C-mutated NSCLC, authors reported a median PFS of 9.3 months (95% CI 7.3–11.8) and median OS of 16.8 months (95% CI 12.7–22.3) [10]. Efficacy has been limited by resistance through increased expression of integrin B4 triggering AKT-mTOR bypass signaling as well as upregulated activation of the WNT-beta-catenin pathway [11]. Table 3 compares divarasib to existing KRAS G12C inhibitors, sotorasib and adagrasib.
The phase III CodeBreak200 compared sotorasib with docetaxel in previously treated NSCLC patients with KRAS G12C mutations. Sotorasib demonstrated a median PFS of 5.6 months (95% CI 4.3–7.8) versus docetaxel of 4.5 months (95% CI 3.0–5.7). However the FDA’s Oncologic Drug Advisory Committee (ODAC) meeting voted that the PFS in CodeBreak200 could not be reliably determined [12]. This was a particularly interesting ODAC, where instead of voting on whether CodeBreaK 200 should be used to convert the accelerated approval of sotorasib to the traditional approval, committee members were asked to vote on whether the primary endpoint of PFS per blinded independent central review (BICR) could be reliably interpreted.
While the CodeBreaK 200 study met its primary endpoint, which was statistically significant, the difference in PFS was small between the two arms and there was no difference in OS. Additionally, the FDA shared concerns on the study conduct. For example, it observed that early dropouts were seen more often with the docetaxel arm, where the investigator assessments of “progression of disease” may have been biased and favored the sotorasib arm, as there were crossover of patients from docetaxel to sotorasib prior to the BICR assessment. Further concerns included lack of adherence to imaging assessment protocols as multiple evaluations appear to have been conducted by BICR to “resolve discrepancies” between investigator and BICR.
The field of KRAS is rapidly evolving. Especially with sotorasib falling short of initial expectations in CodeBreaK 200, this leaves the door open for multiple other KRAS G12C competitors to continue to be developed for use in KRAS G12C NSCLC as well as other tumor types.
In the phase I/II KRYSTAL-1 study, patients with KRAS G12C-mutated NSCLC received adagrasib (Mirati Therapeutics) at 600 mg twice per day (BID). At data cut-off, 116 patients with a median of two prior lines of treatment were enrolled and ORR was 42.9% [13]. The median PFS was 6.5 months (95% CI 4.7–8.4) and median OS was 12.6 months (95% CI 9.2–19.2). The median DOR was 8.5 months (95% CI 6.2–13.8). Adagrasib demonstrated central nervous system (CNS) activity with an ORR of 33% and disease control rate (DCR) 85% in patients with stable, previously treated brain metastases [13, 14]. Adagrasib treatment-related events were common (97.4% of participants), including grade 3 or higher adverse events in 44.8% [14]. The most common toxicities include diarrhea (70.7%), nausea (69.8%), fatigue (59.5%), and vomiting (56.9%). Adagrasib was discontinued owing to adverse events in 6.9% of participants. These findings led to accelerated approval by the FDA in December 2022 for previously treated NSCLC with KRAS G12C mutations.
Currently, sotorasib is priced at US$281.55 per 320 mg pill or US$844.65 per day. Adagrasib is priced at US$147.66 per 200 mg pill or US$885.96 per day. Although pricing information for divarasib is not yet available, additional competitors may drive the price down to decrease the financial toxicity associated with taking newer targeted agents.
Garsorasib or D-1553 is another potent and selective KRAS G12C inhibitor in development. In Phase I results of patients with KRAS G12C-mutated NSCLC, the optimal dose was 600 mg BID [15]. In the dose expansion cohort including 79 patients, 94.5% experienced adverse events including 38% grade 3 or 4 events. In efficacy analysis of 74 patients ORR was 40.5% with median PFS of 8.2 months and median DOR of 7.1 months. In patients with brain metastases, the ORR was 17%. Studies of garsorasib are ongoing.
5 Conclusions
Divarasib is a covalent KRAS G12C inhibitor that in preclinical studies was 5 to 20 times as potent and up to 50 times as selective as sotorasib and adagrasib. In recently reported phase I results, divarasib showed a similar efficacy as other KRAS G12C inhibitors. Although most patients reported toxicities, the majority were grade 1 or 2 gastrointestinal side effects that were manageable with supportive care and reversible.
References
Lee JK, Sivakumar S, Schrock AB, Madison R, Fabrizio D, Gjoerup O, et al. Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. NPJ Precis Oncol. 2022;6(1):91.
Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRASG12C somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384(2):185–7.
Dang CV, Reddy EP, Shokat KM, Soucek L. Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer. 2017;17(8):502–8.
Lindsay CR, Jamal-Hanjani M, Forster M, Blackhall F. KRAS: reasons for optimism in lung cancer. Eur J Cancer. 2018;99:20–7.
Wankhede D, Bontoux C, Grover S, Hofman P. Prognostic role of KRAS G12C mutation in non-small cell lung cancer: a systematic review and meta-analysis. Diagnostics (Basel). 2023;13(19):3043.
Purkey H. Abstract ND11: discovery of GDC-6036, a clinical stage treatment for KRAS G12C-positive cancers. Cancer Res. 2022;82(12_Supplement):ND11.
Sacher A, LoRusso P, Patel MR, Miller WH Jr, Garralda E, Forster MD. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N Engl J Med. 2023;389(8):710–21.
Desai J, Han S-W, Lee J-S, Shaham-Shmueli E, Massarelli E, Cervantes A. Abstract CT029: phase Ib study of GDC-6036 in combination with cetuximab in patients with colorectal cancer (CRC) with KRAS G12C mutation. Cancer Res. 2023;83(8_Supplement):CT029.
Dy GK, Govindan R, Velcheti V, Falchook GS, Italiano A, Wolf J. Long-term outcomes and molecular correlates of sotorasib efficacy in patients with pretreated KRAS G12C-mutated non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J Clin Oncol. 2023;41(18):3311–7.
Iams WT, Balbach ML, Phillips S, Sacher A, Bestvina C, Velcheti V. A multicenter retrospective chart review of clinical outcomes among patients with KRAS G12C mutant non-small cell lung cancer. Clin Lung Cancer. 2023;24(3):228–34.
Mohanty A, Nam A, Srivastava S, Jones J, Lomenick B, Singhal SS. Acquired resistance to KRAS G12C small-molecule inhibitors via genetic/nongenetic mechanisms in lung cancer. Sci Adv. 2023;9(41):eade3816.
Administration, U.F.D. October 5, 2023: Meeting of the Oncologic Drugs Advisory Committee Meeting Announcement. 2023. https://www.fda.gov/advisory-committees/advisory-committee-calendar/october-5-2023-meeting-oncologic-drugs-advisory-committee-meeting-announcement-10052023#event-materials.
Jänne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM. Adagrasib in non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387(2):120–31.
Sabari JK, Velcheti V, Shimizu K, Strickland MR, Heist RS, Singh M. Activity of adagrasib (MRTX849) in brain metastases: preclinical models and clinical data from patients with KRASG12C-mutant non-small cell lung cancer. Clin Cancer Res. 2022;28(15):3318–28.
Li Z, Song Z, Zhao Y, Wang P, Jiang L, Gong Y. D-1553 (Garsorasib), a potent and selective inhibitor of KRAS. J Thorac Oncol. 2023;18(7):940–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
DB declares that she has no conflicts of interest that might be relevant to the contents of this manuscript. MN received consulting fees from Caris Life Sciences, honoraria from AstraZeneca, Daiichi Sankyo, Novartis, Lilly, Pfizer, EMD Serono, Genentech, Regeneron, and BMS. MN is a speaker for Mirati, Takeda, Janssen, and Blueprint Medicine and has received travel support from AnHeart Therapeutics.
Ethics approval and consent to participate
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
DB provided clinical interpretation and drafted and reviewed all versions of the manuscript. MN provided clinical interpretation and drafted and reviewed all versions of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brazel, D., Nagasaka, M. Divarasib in the Evolving Landscape of KRAS G12C Inhibitors for NSCLC. Targ Oncol 19, 297–301 (2024). https://doi.org/10.1007/s11523-024-01055-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-024-01055-y