Abstract
Introduction
Whether mutation status should be used to guide therapy is an important issue in many cancers. We correlated mutation profile in radioiodine-refractory (RAIR) metastatic thyroid cancers (TCs) with patient outcome and response to tyrosine kinase inhibitors (TKIs), and discussed the results with other published data.
Materials and Methods
Outcome in 82 consecutive patients with metastatic RAIR thyroid carcinoma prospectively tested for BRAF, RAS and PI3KCA mutations was retrospectively analyzed, including 55 patients treated with multikinase inhibitors.
Results
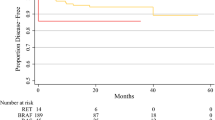
Papillary thyroid carcinomas (PTCs) were the most frequent histological subtype (54.9 %), followed by poorly differentiated thyroid carcinoma [PDTC] (30.5 %) and follicular thyroid carcinoma [FTC] (14.6 %). A genetic mutation was identified in 23 patients (28 %) and BRAF was the most frequently mutated gene (23 %). Median progression-free survival (PFS) on first-line TKI treatment was 14.6 months (95% CI 9.9–18.4). BRAF mutation positively influenced median PFS, both in the entire TKI-treated cohort (median PFS 34.7 months versus 11.6 months; hazard ratio [HR] 0.29; 95% CI 0.09–0.98; p = 0.03) and in the TKI-treated PTC cohort (n = 22) [log-rank p = 0.086; HR 2.95; 95 % CI 0.81–10.70). However, in TKI-treated patients, PDTC histologic subtype was the only independent prognostic factor for PFS identified in the multivariate analysis (HR 2.36; 95% CI 1.01–5.54; p = 0.048).
Conclusion
Patients with BRAF-mutant PTC had a significantly longer PFS than BRAF wild-type when treated with TKIs. However, due to the small number of BRAF-mutant patients, further investigations are required, especially to understand the potential positive effect of BRAF mutations in RAIR TC patients while having a negative prognostic impact in RAI-sensitive PTC patients.





Similar content being viewed by others
Reference
DeLellis R, Lloyd RV, Heitz PU, Eng C (eds). World Health Organization Classification of Tumours. Pathology and genetics of tumours and endocrine organs. Lyon: IARC Press; 2004.
Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990 to 2008. J Natl Cancer Inst 2013;105(15):1096-110.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP et al (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899
Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F et al (2014) Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diab Endocrinol 2:356–358
de la Fouchardiere C, Droz JP (2011) Targeted therapies and thyroid cancer: an update. Anticancer Drugs 22:688–699
Brose MS, Nutting C, Jarzab B, Elisei R, Siena S, Bastholt L et al (2013) Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: the phase III DECISION trial. J Clin Oncol (Meeting Abstracts) 31(4)
Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014;384(9940):319–28.
Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R et al (2015) Dutcus CE, de las HB, Zhu J, Sherman SI: Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630
Cabanillas ME, Waguespack SG, Bronstein Y, Williams MD, Feng L, Hernandez M et al (2010) Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab 95:2588–2595
Hoftijzer H, Heemstra KA, Morreau H, Stokkel MP, Corssmit EP, Gelderblom H et al (2009) Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol 161:923–931
Massicotte MH, Brassard M, Claude-Desroches M, Borget I, Bonichon F, Giraudet AL et al (2014) Tyrosine kinase inhibitor treatments in patients with metastatic thyroid carcinomas: a retrospective study of the TUTHYREF network. Eur J Endocrinol 170:575–582
Xing M (2007) BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28:742–762
Xing M, Haugen BR, Schlumberger M (2013) Progress in molecular-based management of differentiated thyroid cancer. Lancet 381:1058–1069
Nikiforov YE (2011) Molecular analysis of thyroid tumors. Mod Pathol 24(Suppl 2):S34–S43
Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R et al (2007) BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab 92:2840–2843
Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R et al (2008) BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93:3943–3949
Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ et al (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373–6379
Howell GM, Hodak SP, Yip L (2013) RAS mutations in thyroid cancer. Oncologist 18:926–932
Nikiforov YE, Nikiforova MN (2011) Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 7:569–580
Kondo T, Ezzat S, Asa SL (2006) Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 6:292–306
Nikiforova MN, Nikiforov YE (2008) Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn 8:83–95
Ruggeri RM, Campenni A, Baldari S, Trimarchi F, Trovato M (2008) What is new on thyroid cancer biomarkers. Biomark Insights 3:237–252
Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J et al (2003) Ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 21:3226–3235
Wojciechowska-Durczynska K, Krawczyk-Rusiecka K, Cyniak-Magierska A, Zygmunt A, Galecka E, Lewinski A (2010) Relative quantification of PIK3CA gene expression level in fine-needle aspiration biopsy thyroid specimens collected from patients with papillary thyroid carcinoma and non-toxic goitre by real-time RT-PCR. Thyroid Res 3:5
Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM et al (2005) Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab 90:4688–4693
Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S et al (2007) Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 13:1161–1170
Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS et al (2011) PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS One 6:e22769
Schlumberger M, Sherman SI (2012) Approach to the patient with advanced differentiated thyroid cancer. Eur J Endocrinol 166:5–11
Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M et al (2009) Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 69:4885–4893
Xing M (2013) Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501
Mian C, Barollo S, Pennelli G, Pavan N, Rugge M, Pelizzo MR et al (2008) Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf) 68:108–116
Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R et al (2009) Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684
Acknowledgments
Rob Stepney (Medical Writer, Charlbury, UK) contributed to the editing of this paper.
Compliance with Ethical Standards
ᅟ
Conflicts of Interest
Grants, fees and honoraria: Christelle de la Fouchardiere (Roche, Bayer); Anne-Laure Giraudet (Genzyme, travel for meetings); Pierre-Paul Bringuier (Roche)
Board membership: Christelle de la Fouchardiere (Bayer, Sobi, Celgene, Astra-Zeneca).
Olfa Derbel, Myriam Decaussin-Petrucci, Marie-Eve Fondrevelle, Qing Wang, Claire Bournaud-Salinas, Jean-Louis Peix, Jean-Christophe Lifante, Jonathan Lopez, Nadia Oussaid, and Françoise Borson-Chazot have no conflicts of interest to declare.
Funding
This research did not receive any specific grants from any funding agency in the public, commercial, or not-for-profit sector.
Author Contributions
Christelle de la Fouchardiere conceived the study, participated in its design and coordination, and helped draft the manuscript. Françoise Borson-Chazot and Christelle de la Fouchardiere participated in the design of the study. Myriam Decaussin-Petrucci and Marie-Eve Fondrevelle were in charge of pathological analysis. Qing Wang and Pierre-Paul Bringuier carried out the molecular genetic studies and drafted the manuscript, and Nadia Oussaid performed the statistical analysis. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de la Fouchardiere, C., Oussaid, N., Derbel, O. et al. Does Molecular Genotype Provide Useful Information in the Management of Radioiodine Refractory Thyroid Cancers? Results of a Retrospective Study. Targ Oncol 11, 71–82 (2016). https://doi.org/10.1007/s11523-015-0380-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0380-y