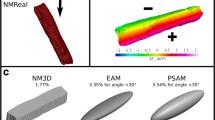
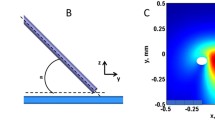
Abstract
External electric fields (E), used for cardiac pacing and defibrillation/cardioversion, induce a spatially variable change in cardiomyocyte transmembrane potential (ΔVm) that depends on cell geometry and E orientation. This study investigates E-induced ΔVm in cardiomyocytes from rats at different ages, which show marked size/geometry variation. Using a tridimensional numerical electromagnetic model recently proposed (NM3D), it was possible: (a) to evaluate the suitability of the simpler, prolate spheroid analytical model (PSAM) to calculate amplitude and location of ΔVm maximum (ΔVmax) for E = 1 V.cm−1; and (b) to estimate the ΔVmax required for excitation (ΔVT) from experimentally determined threshold E values (ET). Ventricular myocytes were isolated from neonatal, weaning, adult, and aging Wistar rats. NM3D was constructed as the extruded 2D microscopy cell image, while measured minor and major cell dimensions were used for PSAM. Acceptable ΔVm estimates can be obtained with PSAM from paralelepidal cells for small θ. ET, but not ΔVT, was higher for neonate cells. ΔVT was significantly greater in the cell from older animals, which indicate lower responsiveness to E associated with aging, rather than with altered cell geometry/dimensions. ΔVT might be used as a non-invasive indicator of cell excitability as it is little affected by cell geometry/size.
Graphical Abstract







Similar content being viewed by others
References
Klee M, Plonsey R (1976) Stimulation of spheroidal cells: the role of cell shape. IEEE Trans Biomed Engin 1976(23):347–354. https://doi.org/10.1109/TBME.1976.324597
Gross D, Loew LM, Webb WW (1986) Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J 50:339–348. https://doi.org/10.1016/s0006-3495(86)83467-1
Sharma V, Tung L (2002) Spatial heterogeneity of transmembrane potential responses of single guinea-pig cardiac cells during electric field stimulation. J Physiol 542:477–492. https://doi.org/10.1113/jphysiol.2001.013197
Oliveira PX, Bassani RA, Bassani JWM (2008) Lethal effect of electric fields on isolated ventricular myocytes. IEEE Trans Biomed Engin 55:2635–2642. https://doi.org/10.1109/tbme.2008.2001135
Nikolski VP, Efimov IR (2005) Electroporation of the heart. Europace 7:S146–S154. https://doi.org/10.1016/j.eupc.2005.04.011
Clementy N, Bodin Bisson A, Teixeira-Gomes AP, Roger S, Angoulvant D, Labas V, Babudy D (2021) The defibrillation conundrum: new insights into the mechanisms of shock-related myocardium injury sustained from a life-saving therapy. Int J Mol Sci 22:5003. https://doi.org/10.3390/ijms22095003
Tung L, Borderies JR (1992) Analysis of electric field stimulation of single cardiac muscle cells. Biophys J 63:371–386. https://doi.org/10.1016/2FS0006-3495(92)81632-6
Krassowska W, Neu JC (1994) Response of a single cell to an external electric field. Biophys J 66:1768–1776. https://doi.org/10.1016/S0006-3495(94)80971-3
Ranjan R, Thakor NV (1995) Electrical stimulation of cardiac myocytes. Ann Biomed Engin 23:812–821. https://doi.org/10.1007/BF02584480
Gomes PAP, Bassani RA, Bassani JWM (2001) Electric field stimulation of cardiac myocytes during postnatal development. IEEE Trans Biomed Engin 48:630–636. https://doi.org/10.1109/10.923781
Valič B, Golzio M, Pavlin M, Schatz A, Faurie C, Gabriel B, Teissié J, Rols MP, Miklavčič D (2003) Effect of electric field induced transmembrane potential on spheroidal cells: theory and experiment. Eur Biophys J 32:519–528. https://doi.org/10.1007/s00249-003-0296-9
Bassani RA, Lima KA, Gomes PAP, Oliveira PX, Bassani JWM (2006) Combining stimulus direction and waveform for optimization of threshold stimulation of isolated ventricular myocytes. Physiol Meas 27:851–863. https://doi.org/10.1088/0967-3334/27/9/008
Goulart JT, Oliveira PX, Bassani JWM, Bassani RA (2012) The influence of cell dimensions on the vulnerability of ventricular myocytes to lethal injury by high-intensity electrical fields. Brazilian J Biomed Eng 28:337–345. https://doi.org/10.4322/rbeb.2012.040
Kotnik T Transmembrane voltage induced by applied electric field. In: Miklavcic, D. (eds.), Handbook of Electroporation. Springer, pp 1–17 Cham. https://doi.org/10.1007/978-3-319-26779-1_8-1
Escobar J, Vaca-González JJ, Guevara JM, Garzón-Alvarado DA (2020) Effect of magnetic and electric fields on plasma membrane of single cells: a computational approach. Engin Rep 2:e12125. https://doi.org/10.1002/eng2.12125
Scuderi M, Dermol-Černe J, Napotnik TB, Chaigne S, Bernus O, Benoist D, Sigg DC, Rems L, Miklavičič D (2023) Characterization of experimentally observed complex interplay between pulse duration, electrical field strength, and cell orientation on electroporation outcome using a time-dependent nonlinear numerical model. Biomolecules 13:727. https://doi.org/10.3390/biom1305072
Pucihar G, Kotnik T, Valič B, Miklavcic D (2006) Numerical determination of transmembrane voltage induced on irregularly shaped cells. Ann Biomed Eng 34:642–652. https://doi.org/10.1007/s10439-005-9076-2
Milan HFM, Bassani RA, Santos LEC, Almeida ACG, Bassani JWM (2019) Accuracy of electromagnetic models to estimate cardiomyocyte membrane polarization. Med Biol Eng Comput 57:2617–2627. https://doi.org/10.1007/s11517-019-02054-2
Papadacci C, Finel V, Provost J, Villemain O, Bruneval P, Gennisson JL, Tanter M, Fink M, Pernot M (2017) Imaging the dynamics of cardiac fiber orientation in vivo using 3D ultrasound backscatter tensor imaging. Sci Rep 7:830. https://doi.org/10.1038/s41598-017-00946-7
Jaeger KH, Edwards AG, Giles WR, Tveito A (2021) From millimeters to micrometers: re-introducing myocytes in models of cardiac electrophysiology. Front Physiol 12. https://doi.org/10.3389/fphys.2021.763584
Rupp LC, Bergquist JA, Zenger B, Gillette K, Narayan A, Tate JD, Plank G, MacLeod RD (2021) The role of myocardial fiber direction in epicardial activation patterns via uncertainty quantification. 2021 Computing in Cardiology (CinC), Brno, Czech Republic, pp 1–4. https://doi.org/10.23919/CinC53138.2021.9662950
Mountris KA, Pueyo E (2022) A meshless fragile points method for rule-based definition of myocardial fiber orientation. Computer Meth Progr Biomed 226:107164. https://doi.org/10.1016/j.cmpb.2022.107164
Frey W, White JA, Price RO, Blackmore PF, Joshi RP, Nuccitelli R, Beebe SJ, Schoenbach KH, Kolb JF (2006) Plasma membrane voltage changes during nanosecond pulsed electric field exposure. Biophys J 90:3608–3615. https://doi.org/10.1529/biophysj.105.072777
Yechiel E, Barenholz Y (1985) Relationships between membrane lipid composition and biological properties of rat myocytes. Effects of aging and manipulation of lipid composition. J Biol Chem 260:9123–9131. https://doi.org/10.1016/S0021-9258(17)39339-0
Mitcheson JS, Hancox JC, Levi AJ (1996) Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflugers Arch 431:814–827. https://doi.org/10.1007/s004240050073
Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, Chen-Izu Y, Izu LT, Balke CW (2008) Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol 93:370–382. https://doi.org/10.1113/expphysiol.2007.040659
Hammer K, Ruppenthal S, Viero C, Scholz A, Edelmann L, Kaestner L, Lipp P (2010) Remodelling of Ca2+ handling organelles in adult rat ventricular myocytes during long term culture. J Mol Cell Cardiol 49:427–437. https://doi.org/10.1016/j.yjmcc.2010.05.010
Bassani RA, Bassani JWM (2002) Contribution of Ca2+ transporters to relaxation in intact ventricular myocytes from developing rats. Am J Physiol Heart Circ Physiol 282:H2406–H2413. https://doi.org/10.1152/ajpheart.00320.2001
Penna LB, Bassani RA (2010) Increased spontaneous activity and reduced inotropic response to catecholamines in ventricular myocytes from footshock-stressed rats. Stress 13:73–82. https://doi.org/10.3109/10253890902951778
Milan HFM, Bassani RA, Bassani JWM (2015) Testing electrode suitability for field stimulation of high-threshold biological preparations. Res Biomed Eng 31:273–276. https://doi.org/10.1590/2446-4740.0718
Satoh H, Delbridge LMD, Blatter LA, Bers DM (1996) Surface: volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and development effects. Biophys J 70:1494–1504. https://doi.org/10.1016/2FS0006-3495(96)79711-4
Geuzaine C, Remacle JF (2009) A three-dimensional finite element mesh generator with built-in pre- and post-processing facilities. Int J Numer Method Engin 79:1309–1331. https://doi.org/10.1002/nme.2579
Cooklin M, Wallis WRJ, Sheridan DJ, Fry CH (1997) Changes in cell-to-cell electrical coupling associated with left ventricular hypertrophy. Circ Res 80:765–771. https://doi.org/10.1161/01.RES.80.6.765
Fry CH, Salvage SC, Manazza A, Dupont E, Labeed FH, Hughes MP, Jabr RI (2012) Cytoplasm resistivity of mammalian atrial myocardium determined by dielectrophoresis and impedance methods. Biophys J 103:2287–2294. https://doi.org/10.1016/2Fj.bpj.2012.10.023
Pinheiro J and Bates D (2000) Statistics and computing: mixed-effects models in S and S-PLUS. Springer-Verlag New York, Inc. Ed. 1, p 528. https://doi.org/10.1007/b98882
Zuur AF, Leno EN, Walker N, Saveliev AA, Smith GM (2009) Statistics for biology and health: mixed effects models and extensions in ecology with R. Ed 1, Springer, New York, pp 574. https://doi.org/10.1007/978-0-387-87458-6
Crawley MJ (2013) The R Book. John Wiley & Sons, Ltd, Chichester, United Kingdom, ed. 2, 1051 pp, ISBN: 978–0–470–97392–9. http://www.bio.ic.ac.uk/research/mjcraw/therbook/index.htm
James G, Witten D, Hastie T, Tibshirani R (2013) An introduction to statistical learning: with applications in R. Springer, New York. https://doi.org/10.1007/978-1-4614-7138-7
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Efron E, Hastie T (2016) Computer age statistical inference: algorithms, evidence, and data science. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781316576533
Davison AC, Hinkley DV (1997) Boostrap methods and their applications. Cambridge University Press, Cambridge
Canty A, Ripley B (2017) Boot: bootstrap R (S-Plus) Functions. R package version 1.3–20. https://cran.r-project.org/web/packages/boot/citation.html
Howlett SE (2010) Age-associated changes in excitation-contraction coupling are more prominent in ventricular myocytes from male rats than in myocytes from female rats. Am J Physiol Heart Circ Physiol 298:H659–H670. https://doi.org/10.1152/ajpheart.00214.2009
Awad AB, Clay SW (1982) Age-dependent alterations in lipids and function of rat heart sarcolemma. Mech Ageing Dev 19:333–342. https://doi.org/10.1016/0047-6374(82)90017-3
Nagatomo T, Sasaki M, Konishi T (1984) Differences in lipid composition and fluidity of cardiac sacrolemma prepared from newborn and adult rabbits. Biochem Med 32:122–131. https://doi.org/10.1016/0006-2944(84)90014-0
Kilborn MJ, Fedida D (1990) A study of the developmental changes in outward currents of rat ventricular myocytes. J Physiol 430:37–60. https://doi.org/10.1113/2Fjphysiol.1990.sp018280
Wahler GM, Dollinger SJ, Smith JM, Flemal KL (1994) Time course of postnatal changes in rat heart action potential and in transient outward current is different. Am J Physiol Heart Circ Physiol 267:H1157–H1166. https://doi.org/10.1152/ajpheart.1994.267.3.H1157
Lieber SC, Aubry N, Pain J, Diaz G, Kim S-J, Vatner SF (2004) Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 287:H645–H651. https://doi.org/10.1152/ajpheart.00564.2003
Stocker P, Bennett ES (2006) Differential sialylation modulates voltage-gated Na+ channel gating throughout the developing myocardium. J Gen Physiol 127:253–265. https://doi.org/10.1085/jgp.200509423
Oshiyama NF, Pereira AHM, Cardoso AC, Franchini KG, Bassani JWM, Bassani RA (2022) Developmental differences in myocardial transmembrane Na+ transport: implications for excitability and Na+ handling. J Physiol 600:2651–2667. https://doi.org/10.1113/JP282661
Kishida H, Surawicz B, Fu LT (1979) Effect of K+ and K+-induced polarization on (dV/dt)max, threshold potential, and membrane input resistance in guinea pig and cat ventricular myocardium. Circ Res 44:800–814. https://doi.org/10.1161/01.RES.44.6.800
Golod DA, Kumar R, Joyner RW (1998) Determinants of the action potential initiation in isolated rabbit and ventricular myocytes. Am J Physiol Heart Circ Physiol 43:H1902–H1913. https://doi.org/10.1152/ajpheart.1998.274.6.H1902
Cheng DD-L, Tung L, Sobie E (1999) Nonuniform responses of transmembrane potential during electric field stimulation of single cardiac cells. Am J Physiol Heart Circ Physiol 277:H351–H362. https://doi.org/10.1152/ajpheart.1999.277.1.H351
Gomes PAP, Galvão KD, Mateus EF (2002) Excitability of isolated hearts from rats during postnatal development. J Cardiovasc Electrophysiol 13:355–360. https://doi.org/10.1046/j.1540-8167.2002.00355.x
Kaufmann SG, Westenbroek RE, Zechner C, Maass AH, Bischoff S, Muck J, Wischmeyer E, Scheuer T, Maier SKG (2010) Functional protein expression of multiple sodium channel α- and β-subunit isoforms in neonatal cardiomyocytes. J Mol Cell Cardiol 48:261–269. https://doi.org/10.1016/j.yjmcc.2009.04.017
Wei JY, Spurgeon HA, Lakatta EG (1984) Excitation-contraction in rat myocardium: alterations with adult aging. Am J Physiol Heart Circ Physiol 246:H784–H791. https://doi.org/10.1152/ajpheart.1984.246.6.h784
Dibb K, Eisner D, Isenberg G, Rueckschloss, Trafford A (2004) Alterations in the properties of the cardiac inward rectifier potassium channel IK1 in aged sheep. J Physiol 557P:C9. https://www.physoc.org/abstracts/alterations-in-the-properties-of-the-cardiac-inward-rectifier-potassium-channel-ik1-in-aged-sheep/
Cooper S, Jones S (2015) 201 Age-associated decline in Nav1.4 and Nav1.5 isoforms of the sodium channel in rat heart. Heart 101(suppl 4):A1–A136. https://doi.org/10.1136/heartjnl-2015-308066.201
Lim WY, Prabhu S (2019) Implantable cardiac electronic devices in the elderly population. Arrh Electrophysiol Rev 8:143–146. https://doi.org/10.15420/aer.2019.3.4
Barra S, Providência R, Paiva L, Heck P, Agarwal S (2015) Implantable cardioverter-defibrillators in the elderly: rationale and specific age-related considerations. Europace 17:174–186. https://doi.org/10.1093/europace/euu296
Acknowledgements
The authors are indebted to Ms. Elizângela S. Oliveira and Mr. Renato S. Moura (CEB/UNICAMP) for the technical support, and to Dr. Lynn M. Johnson (Cornell Univerity Statistical Consulting Unit) for the statistical support.
Funding
São Paulo Research Foundation (FAPESP, Proc. 2008/54795–6 and 2013/05441–5), Brazilian National Council for Scientific and Technological Development (CNPq, Proc. 302996/2011–7, 30408/2022-0 and 203312/2014–7), Brazilian Ministry of Health and Funding Authority for Studies and Projects (Finep: 0441/12 - 01.13.0214.00), and Coordination for the Improvement of Higher Education Personnel (CAPES; scholarship to HFMM and AAA).
Author information
Authors and Affiliations
Contributions
JWMB, RAB, and HFMM were responsible for the conception of the study. HFMM and AAA performed the experiments, the calculations, and the statistical analysis. All authors participated in the interpretation and revision of the results. HFMM, AAA, and RAB elaborated the first version of the manuscript, which was revised and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
Protocols of animal care and use were approved by the institutional Committee of Ethics in Animal Use (CEUA/IB/UNICAMP; process numbers 2941-1D, 2942-1D, 3009-1A, 3281-1 K and 4093-1G).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Cardiac stimulation with electric fields (E) is used to treat arrhythmias.
• During development and aging, the size and shape of cardiac cells change, which may alter their electrical response to E.
• Experiments and computer simulation were used to predict membrane polarization by E.
• The lowest E intensity required for excitation depends on cell geometry and was greater in neonates.
• The membrane depolarization required for excitation was greater in cells from old rats only.
Supplementary Information
ESM 1
(DOCX 725 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Milan, H.F.M., Almazloum, A.A., Bassani, R.A. et al. Membrane polarization at the excitation threshold induced by external electric fields in cardiomyocytes of rats at different developmental stages. Med Biol Eng Comput 61, 2637–2647 (2023). https://doi.org/10.1007/s11517-023-02868-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-023-02868-1