Abstract
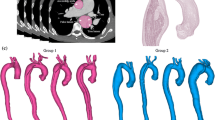
Residual type B aortic dissection was numerically investigated to highlight the contribution of biomechanical parameters to the pathology’s evolution. Patient-specific geometries from cases involving both favorable and unfavorable evolution were modeled to assess their hemodynamic features. This original approach was supported by a longitudinal study confirming the association between morphological changes, hemodynamic features, adverse clinical outcomes, and CT-angioscan observations on the same patient. Comparing one patient with unfavorable evolution with one with favorable one, we identify potential biomechanical indicators predictive of unfavorable evolution: (i) a patent false lumen with a flow rate above 50% of inlet flow rate; (ii) high wall shear stress above 18 Pa at entry tears, and above 10 Pa at some regions of the false lumen wall; (iii) low time-averaged wall shear stress in distal false lumen below 0.5 Pa; (iv) vortical structure dynamics. Although these comparisons could only be conducted on 2 patients and need to be confirmed by a larger number of cases, our findings point to these hemodynamic markers as possible candidates for early evaluation of the pathology’s evolution towards an unfavorable scenario.

Correlation between hemodynamics index and thrombus initiation for unfavorable case. ET2 and ET3 are entry tear numbers 2 and 3 respectively. WSS is wall shear stress. TAWSS is time average shear stress








Similar content being viewed by others
References
Ab Naim WN, Ganesan PB, Sun Z, Chee KH, Hashim SA, Lim E (2014) A perspective review on numerical simulations of hemodynamics in aortic dissection. The Scientific World Journal, 652520–12
Ab Naim WN, Ganesan PB, Sun Z, Osman K, Lim E (2014) The impact of the number of tears in patient-specific stanford type B aortic dissecting anurysm: CFD simulation. J Mech Med Biol 14 (2):1450017
Ab Naim WN, Ganesan PB, Sun Z, Liew YM, Qian Y, Lee C -J, Jansen S, Hashim SA, Lim E (2016) Prediction of thrombus formation using vortical structures presentation in stanford type b dissectio: a preliminary study using CFD approach. Appl Math Model 40:3115–3127
Alimohammadi M, Agu O, Balabani S, Diaz-Zuccarini V (2014) Development of a patient-specific simulation tool to analyse aortic dissections: assessment of mixed patient-specific flow and pressure boundary conditions. Med Eng Phys 36:275–284
Alimohammadi M, Pichardo-Almarza C, Agu O, Diaz-Zuccarini V (2016) Development of a patient-specific multi-scale model to understand atherosclerosis and calcification locations: comparison with in vivo data in aortic dissection. Front Physio 7:238
van Bakel TM, Romarowski RM, Morganti S, van Herwaarden JA, Moll FL, de Baufort HW, Marrocco-Trishitta MM, Secchi F, Conti M, Auricchio F, Trimarchi S (2018) Blood flow after endovascular repair in the aortic arch: a computational analysis. Aorta 6:81–87
Bäumler K, Vedula V, Sailer AM, Seo J, Chiu P, Mistelbauer G, Chan FP, Fischbein MP, Marsden AL, Fleischmann D (2020) Fluid structure interaction simulations of patient specific aortic dissection. Biomech Model Mechan. https://doi.org/10.1007/s10237-020-01294-8
Berguer R, Parodi JC, Schlicht M, Khanafer K (2015) Experimental and clinical evidence supporting septectomy in the primary treatment of acute type b thoracic aortic dissection. Ann Vasc Surg 29:167–173
Biasetti J, Hussain F, Gasser TC (2011) Blood flow and coherent vortices in the normal and aneurysmatic aortas: a fluid dynamical approach to intra-luminal thrombus formation. J R Soc Interface 8:1449–1461
Balabani M, Bonfanti S, Greenwood JP, Puppala S, Homer-Vanniasinkam S, Diaz-Zuccarini V (2017) Computational tools for clinical support: a multi-scale compliant model for haemodynamic simulations in an aortic dissection based on multi-modal imaging data. J R Soc Interface 14:20170632
Carnevale D, Lembo G, Frati G (2011) Chronic Type A aortic dissection: could surgical intervention be guided by molecular markers ? J Cell Mol Med 15(7):1615–1619
Chen D, Müller-Eschner M, Kotelis D, Böckler D, Ventikos Y, von Tengg-Kobligk H (2013) A longitudinal study of type B aortic dissection and endovascular repair scenarios: computational analysis. Med Eng Phys 35:1320–1330
Cheng Z, Riga C, Chan J, Hamady M, Wood NB, Cheshire NJ, Xu Y, Gibbs RGJ (2013) Initial findings and potential applicability of computational simulation of the aorta in acute type b dissection. J Vasc Surg 57:35–43
Cheng Z, Kidher E, Jarral OA, O’Regan DP, Wood NB, Athanasiou T, Xu XY (2015) Assessment of hemodynamic conditions in the aorta following root replacement with composite valve-conduit graft. Ann Biomed Eng 44(5):1392–1404
Cyron C, Humphrey J (2017) Growth and remodeling of load-bearing biological soft tissues. Adv Biomech: From Found Appl 52:645–664
Deplano V, Guivier-Curien C, Bertrand E (2016) 3D analysis of vortical structures in an abdominal aortic aneurysm by stereoscopic PIV. Exp Fluids 57:167
Deplano V, Boufi M, Gariboldi V, Loundo AD, D’Journo XB, Cautela J, Djemli A, Alimi Y (2019) Mechanical characterisation of human ascending aorta dissection. J Biomech 94:138–146
Doyle BJ, Norman PE (2016) Computational biomechanics in thoracic aortic dissection: today’s approaches and tomorrow’s opportunities. Ann Biomed Eng 44(1):71–83
Ergin MA, Phillips RA, Galla JD, Lansman SL, Mendelson DS, Quintana CS, Griepp RB (1994) Significance of distal false lumen after type a dissection repair. Ann Thorac Surg 57:820– 825
Fattori R, Bacchi-Reggiani L, Bertaccini P, Napoli G, Fusco F, Longo M, Pierangeli A, Gavelli G (2000) Evolution of aortic dissection after surgical repair. Am J Cardiol 86:868–872
Fattouch K, Sampognaro R, Navarra E, Caruso M, Pisano C, Coppola G, Speziale G, Ruvolo G (2009) Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 88:1244–1250
Fung YC (1996) Biomechanics. Circulation. Springer, New York
Geirsson A, Bavaria JE, Swarr D, Keane MG, Woo YJ, Szeto WY, Pochettino A (2007) Fate of the residual distal and proximal aorta after acute type a dissection repair using a contemporary surgical reconstruction algorithm. Ann Thorac Surg 84:1955–1964
Guzzardi DG, Barker AJ, van Ooij P, Malaisrie SC, Puthumana JJ, Belke DD, Mewhort HE, Svystonyuk DA, Kang S, Verma S, Collins J, Carr J, Bonow RO, Markl M, Thomas JD, McCarthy PM, Fedak PW (2015) Valve-related hemodynamics mediate human bicuspid aortopathy. J Am Coll Cardiol 66:892– 900
Halstead JC, Meier M, Etz C, Spielvogel D, Bodain C, Wurm M, Shahani R, Griepp RB (2007) The fate of the distal aorta after repair of acute type a aortic dissection. J Thorac Cardiov Sur 13:127–135
Hellums JD, Peterson DM, Stathopoulos NA, Moake JL, Giorgio TD (1987) Studies on the mechanisms of shear-induced platelet activation. In: Hartmann A, et Kuschinsky W (eds) Cerebral Ischemia and Hemorheology. Springer, Berlin, pp 80–89
Khannous F (2020) Modélisation biomécanique et pathologies de l’aorte. Suivi clinique dans le contexte de la dissection aortique de type B résiduelle. PhD Thesis Aix-Marseille Université
Leuprecht A, Perktold K (2001) Computer simulation of non-newtonian effects on blood flow in large arteries. Comput Method Biomech Biomed Eng 4:149–163
Lorensen WE, Cline HE (1987) Marching cubes: a high resolution 3d surface construction algorithm. Comp Graph 21:163– 169
Lorenzo-Ginori JV, Orozco-Morales R (2013) Cell Microscopy Imaging: A Review on Digital Image Processing Applications. Monograph, Feijóo Ed. ISBN 978-959-250-895-8
Menichini C, Cheng Z, Gibbs RG, Xu XY (2016) Predicting false lumen thrombosis in patient-specific models of aortic dissection. J R Soc Interface 13:20160759–1–11
Menichini C, Xu XY (2016) Mathematical modeling of thrombus formation in idealized models of aortic dissection: initial findings and potential applications. J Math Biol 73:1205–1226
Menichini C, Cheng Z, Gibbs RG, Xu XY (2018) A computational model for false lumen thrombosis in type b aortic dissection following thoracic endovascular repair. J Biomech 66:36–43
Mousavi SJ, Farzaneh S, Avril S (2019) Patient-specific predictions of aneurysm growth and remodeling in the ascending thoracic aorta using homogenized constrained mixture model. Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-019-01184-8
Olufsen MS, Peskin CS, Kim WY, Pedersen EM, Nadim A, Larsen J (2000) Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann Biomed Eng 28:1281–1299
Qiao Y, Fan J, Ding Y, Zhu T, Luo K (2020) A primary computational fluid dynamics study of pre- and post-TEVAR with intentional left subclavian artery coverage in a type B aortic dissection. J Biomech Eng 141:111002–1
Rinaudo A, D’Ancona G, Lee JJ, Pilato G, Amaducci A, Baglini R, Follis F, Pilato M, Salvatore P (2014) Predicting outcome of aortic dissection with patent false lumen by computational flow analysis. Cardiovasc Eng Techn 5(2):176–188
Ruggeri ZM, Mendolicchio GL (2007) Adhesion mechanism in platelet function. Circ Res 100:1673–1685
Shang EK, Nathan DP, Fairman RM, Bavaria JE, Gorman RC, Gorman IIIJH, Jackson BM (2015) Use of computational fluid dynamics studies in predicting aneurysmal degeneration of acute type b aortic dissections. J Vasc Surg 62:279–284
Song S-W, Chang B-C, Cho B-K, Yi G, Youn Y-N, Lee S, Yoo K-J (2010) Effects of partial thrombosis on distal aorta after repair of acute debakey type i aortic dissection. The J Thorac Cardiovasc Surg 139:841–847
Tsai M-T, Wu H-Y, Roan J-N, Tsai Y-S, Hsieh PC, Yang Y-J, Luo C-Y (2014) Effect of false lumen partial thrombosis on repaired acute type a aortic dissection. J Thorac Cardiovasc Surg 148:2140–2146
Tse KM, Chiu P, Lee HP, Ho P (2011) Investigation of hemodynamics in the development of dissecting aneurysm within patient-specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J Biomech 44:827–836
Xu H, Piccinelli M, Leshnower BG, Lefieux A, Taylor WR, Veneziani A (2018) Coupled morphological hemodynamic computational analysis of type b aortic dissection: a longitudinal study. Ann Biomed Eng 46:927–939
Zierer A, Voeller RK, Hill KE, Kouchoukos NT, Damiano RJ, Moon MR (2007) Aortic enlargement and late reoperation after repair of acute type a aortic dissection. Ann Thorac Surg 84:479–487
Zhou J, Adrian RJ, Balachandar S, Kendall TM (1999) Mechanisms for generating coherent packets of hairpin vortices in channel flow. J Fluid Mech 387:353–396
Acknowledgements
The authors thank Marjorie Sweetko for English language revision.
Funding
Labex MEC ANR-11-LABX-0092 and CNRS
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Approval number 2019-48
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Windkessel model implementation
Appendix: Windkessel model implementation
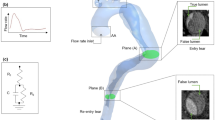
This reduced model describes hydraulic systems through an electrical mechanical analogy where pressure (P) and flow rate (Q) are respectively analogous to voltage and current intensity. This led to the following equation:
with R1 and R2 being the proximal and distal resistance, respectively, and C being the capacitance.
The instantaneous pressure at each outlet was then expressed by approximating the derivative terms using the Euler backward scheme, as follows:
The model parameters R1, R2 and C for each outlet were identified through an optimization procedure using a genetic algorithm and Matlab software. The optimization problem consisted in finding \({R_{1}^{i}}\), \({R_{2}^{i}}\), and Ci for each outlet i that minimize the mismatch between the computed (comp) and the targeted (obj) pressure values. This mismatch Mi is termed the objective function.
Pressure values corresponding to the hypotensive therapy prescribed to the patient were targeted to obtain physiological pressure values P ∈ [80, 120] mmHg with Pmax,obj = 120 mmHg, Pmin,obj = 80 mmHg and Pmean,obj = 100 mmHg.
Two constraints were added
-
1.
Pressure curves must be periodic: Pt= 0 = Pt=T
-
2.
Equation 1 must be verified in the frequency domain when \(\omega \rightarrow 0\) so \(\frac {\bar {P}}{\bar {Q}}=R_{1}+R_{2}\).
The global optimization procedure was inspired by the work of [4]. The first estimation
of flow rate (shapes and values) was obtained through an independent CFD simulation with zero-pressure at each outlet. This has the merit of providing flow rate curves compatible with the morphology of the patients. These curves were then used for a first estimation of the Windkessel parameters using optimization procedure implemented in Matlab software based on genetic algorithm. The targets (pressure values corresponding to anti-hypertensive treatment) were approximated within a tolerance of 10− 16, the optimization result was also evaluated through the Pearson correlation Coefficient r2 which always exceeded 0.99 for each optimization and each outlet. A new simulation was then carried out with Windkessel models defined at each outlet with the estimated parameters. This helped update the flow rate curves that were used again in the optimization procedure (new CFD simulation plus genetic algorithm) and so on until the convergence condition was met. At the end of a step, the objective function (Eq. 3) was assessed for each outlet using the computed pressure values. Their sum was then compared to its value from the previous iteration. If it decreased (meaning that there was still room for minimizing the difference between the computed values and the targeted values) a new optimization iteration was made, else we stopped the procedure and the last parameters estimated were used for the numerical simulations.This procedure is summed up in Fig. 9.
When optimization procedure was ended, that was after several iterations and several CFD simulations, new simulations were carried out, for the current study, with the set of optimal parameters for the Windkessel models at each outlet. Table 2 summarizes the final parameter values of Windkessel model.
Table 3 shows the percentage of flow rate at outlets in relation to inlet one obtained, using Windkessel models at the outlets during simulations. The obtained values are in the range of physiological ones in vivo recorded [6].
Rights and permissions
About this article
Cite this article
Fatma, K., Carine, GC., Marine, G. et al. Numerical modeling of residual type B aortic dissection: longitudinal analysis of favorable and unfavorable evolution. Med Biol Eng Comput 60, 769–783 (2022). https://doi.org/10.1007/s11517-021-02480-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02480-1