Abstract
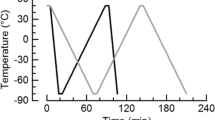
This work is focused on the study of freeze-thaw behavior of double (W1/O/W2) emulsions by the application of a cooling-heating-cooling cycle by differential scanning calorimetry. Emulsions were prepared with sodium caseinate as hydrophilic emulsifier in outer aqueous (W2) phase and sunflower oil (SO) and/or interesterified vegetable fat (VF) plus polyglycerol polyricinoleate as lipophilic emulsifier in lipid phase. The effects of sodium chloride concentration in inner aqueous (W1) phase and solid fat content in lipid phase were analyzed. In all cases, W1 phase froze at lower temperature than W2 phase because higher undercooling was required for the crystallization of inner water droplets. In the absence of VF, the system with low salt concentration showed water diffusion from undercooled W1 phase to frozen W2 phase at the first cooling stage, due to the lower vapor pressure of ice. However, at the second cooling stage, this phenomenon was not observed and partial recovery of inner water was detected, probably because of an osmotic effect. The increase of salt concentration allowed a higher retention of inner water after freezing of W2 phase, attributed to the lowered vapor pressure of W1 phase. The loss of inner water was also restrained or prevented by the presence of VF in lipid phase due to the immobilization of water droplets within a fat crystal network. The crystallization behavior of SO was related to inner water quantity and VF content. This study could be useful for the formulation of W1/O/W2 emulsions with enhanced freeze-thaw stability.





Similar content being viewed by others
References
B. de Cindio, D. Cacace, Int. J. Food Sci. Technol. 30, 505–514 (1995)
A. Benichou, A. Aserin, N. Garti, Colloids Surf. A Physicochem. Eng. Asp. 294(1-3), 20–32 (2007)
M. Bonnet, M. Cansell, A. Berkaoui, M.H. Ropers, M. Anton, F. Leal-Calderon, Food Hydrocoll. 23(1), 92–101 (2009)
A.L. Márquez, J.R. Wagner, J. Texture Stud. 41, 651–671 (2010)
J. O’Regan, D.M. Mulvihill, Food Res. Int. 43(1), 224–231 (2010)
T.A. Comunian, M. Thomazini, A.J. Gouvêa Alves, F.E. de Matos Junior, J.C. de Carvalho Balieiro, C.S. Favaro-Trindade, Food Res. Int. 52(1), 373–379 (2013)
I. Klojdová, J. Štětina, Š. Horáčková, Chem. Eng. Technol. 42(4), 715–727 (2019)
G. Muschiolik, E. Dickinson, Compr. Rev. Food Sci. Food Saf. 16(3), 532–555 (2017)
R. Mezzenga, B.M. Folmer, E. Hughes, Langmuir 20, 3574–3582 (2004)
F. Leal-Calderon, S. Homer, A. Goh, L. Lundin, Food Hydrocoll. 27(1), 30–41 (2012)
N. Garti, A. Aserin, I. Tiunova, H. Binyamin, J. Am. Oil Chem. Soc. 76(3), 383–389 (1999)
S. Frasch-Melnik, F. Spyropoulos, I.T. Norton, J. Colloid Interface Sci. 350(1), 178–185 (2010)
E.C. Rojas, K.D. Papadopoulos, Langmuir 23(13), 6911–6917 (2007)
A. Kovács, I. Csóka, M. Kónya, E. Csányi, A. Fehér, I. Erős, J. Therm, Anal. Calorim. 82(2), 491–497 (2005)
D. Clausse, F. Gomez, I. Pezron, L. Komunjer, C. Dalmazzone, Adv. Colloid Interf. Sci. 117(1-3), 59–74 (2005)
A. Schuch, K. Köhler, H.P. Schuchmann, J. Therm, Anal. Calorim. 111, 1881–1890 (2013)
S. Charoenrein, D.S. Reid, Thermochim. Acta 156, 373–381 (1989)
D. Clausse, F. Gomez, C. Dalmazzone, C. Noik, J. Colloid Interface Sci. 287(2), 694–703 (2005)
S. Ghosh, D. Rousseau, J. Colloid Interface Sci. 339(1), 91–102 (2009)
E. Magnusson, C. Rosén, L. Nilsson, Food Hydrocoll. 25(4), 707–715 (2011)
S. Matsumoto, W.W. Kang, J. Dispers, Sci. Technol. 10, 455–482 (1989)
M.P. Pérez, J.R. Wagner, A.L. Márquez, Eur. J. Lipid Sci. Technol. 119(10), 1600447 (2017)
D.D. Jenkins, Phys. Educ. 17(2), 82–83 (1982)
A.L. Márquez, M.P. Pérez, J.R. Wagner, J. Am. Oil Chem. Soc. 90(4), 467–473 (2013)
M.P. Pérez, M.F. Tesei, J.R. Wagner, A.L. Márquez, J. Texture Stud. 45, 396–407 (2014)
A.L. Márquez, A. Medrano, L.A. Panizzolo, J.R. Wagner, J. Colloid Interface Sci. 341(1), 101–108 (2010)
M.P. Aronson, M.F. Petko, J. Colloid Interface Sci. 159(1), 134–149 (1993)
D. Clausse, I. Pezron, L. Komunjer, Colloids Surf. A Physicochem. Eng. Asp. 152(1-2), 23–29 (1999)
L. Potier, S. Raynal, M. Seiller, J.L. Grossiord, D. Clausse, Thermochim. Acta 204(1), 145–155 (1992)
T. Hatakeyama, H. Hatakeyama, K. Nakamura, Thermochim. Acta 253, 137–148 (1995)
A.B. Arons, C.F. Kientzler, Trans. Am. Geophys. Union 35(5), 722–728 (1954)
M. Cerdeira, S. Martini, R.J. Candal, M.L. Herrera, J. Am. Oil Chem. Soc. 83(6), 489–496 (2006)
K. Shimamura, S. Ueno, Y. Miyamoto, K. Sato, Cryst. Growth Des. 13(11), 4746–4754 (2013)
M.A. Fontenele Domingues, A.P. Badan Ribeiro, M.C. Chiu, L.A. Guaraldo Gonçalves, LWT-Food Sci. Technol. 62(1), 122–130 (2015)
N. Garti, H. Binyamin, A. Aserin, J. Am. Oil Chem. Soc. 75(12), 1825–1831 (1998)
D.J. McClements, S.R. Dungan, J.B. German, C. Simoneau, J.E. Kinsella, J. Food Sci. 58(5), 1148–1151 (1993)
K. Sato, Chem. Eng. Sci. 56(7), 2255–2265 (2001)
E. Fredrick, P. Walstra, K. Dewettinck, Adv. Colloid Interf. Sci. 153(1-2), 30–42 (2010)
M.B. Munk, M.L. Andersen, Eur. J. Lipid Sci. Technol. 117(10), 1627–1635 (2015)
S.M. Hodge, D. Rousseau, J. Am. Oil Chem. Soc. 82(3), 159–164 (2005)
Acknowledgements
The authors acknowledge the financial support of Agencia Nacional de Promoción Científica y Tecnológica (FONCyT; grant number: PICT-2016-2699); and Universidad Nacional de Quilmes (Program I + D; grant number: 53/1037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Márquez, A.L., Wagner, J.R. Analysis of Freeze-Thaw Behavior of Double (W1/O/W2) Emulsions by Differential Scanning Calorimetry: Effects of Inner Salt Concentration and Solid Fat Content. Food Biophysics 16, 98–108 (2021). https://doi.org/10.1007/s11483-020-09653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-020-09653-9