Abstract
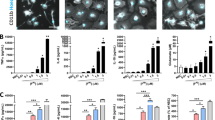
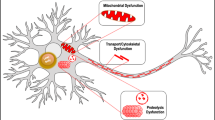
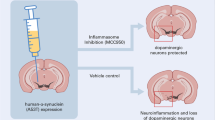
Parkinson's disease (PD) is recognized as the most common neurodegenerative movement disorder and results in debilitating motor deficits. The accumulation and spread of neurotoxic synuclein aggregates in the form of Lewy bodies is a key pathological feature of PD. Chronic activation of the NLRP3 inflammasome by protein aggregates is emerging as a major pathogenic mechanism in progressive neurodegenerative disorders and is considered an important therapeutic target. Recently the ketone body, β-hydroxy butyrate (BHB), was shown to efficiently inhibit the NLRP3 inflammasome in macrophages, and in vivo models of inflammatory disease. Furthermore, BHB can readily cross the blood brain barrier suggesting that it could have therapeutic benefits for the management of PD. In this study, we evaluated if BHB could inhibit chronic microglial inflammasome activation induced by pathological fibrillar synuclein aggregates. Interestingly, we found that BHB treatment almost completely blocked all aspects of inflammasome activation and pyroptosis induced by ATP and monosodium urate (MSU) crystals, consistent with previously published reports in macrophages. Surprisingly however, BHB did not inhibit inflammasome activation and release of IL-1β or caspase-1 induced by synuclein fibrils. Our results demonstrate that BHB does not block the upstream pathways regulating inflammasome activation by synuclein fibrils and suggest that synuclein mediated inflammasome activation proceeds via distinct mechanisms compared to traditional NLRP3 activators such as ATP and MSU.


Similar content being viewed by others
References
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Bezbradica JS, Coll RC, Schroder K (2017) Sterile signals generate weaker and delayed macrophage NLRP3 inflammasome responses relative to microbial signals. Cell Mol Immunol 14:118–126
Coll RC et al (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255
Gordon R, Hogan CE, Neal ML, Anantharam V, Kanthasamy AG, Kanthasamy A (2011) A simple magnetic separation method for high-yield isolation of pure primary microglia. J Neurosci Methods 194:287–296
Gustin A, Kirchmeyer M, Koncina E, Felten P, Losciuto S, Heurtaux T, Tardivel A, Heuschling P, Dostert C (2015) NLRP3 Inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS One 10:e0130624
Gustot A, Gallea JI, Sarroukh R, Celej MS, Ruysschaert JM, Raussens V (2015) Amyloid fibrils are the molecular trigger of inflammation in Parkinson's disease. Biochem J 471:323–333
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT (2013) NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer's disease. Nat Immunol 16:229–236
Herva ME, Zibaee S, Fraser G, Barker RA, Goedert M, Spillantini MG (2014) Anti-amyloid compounds inhibit alpha-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA). J Biol Chem 289:11897–11905
Jakobs C, Bartok E, Kubarenko A, Bauernfeind F, Hornung V (2013) Immunoblotting for active caspase-1. Methods Mol Biol 1040:103–115
Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL (2000) D-beta-hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A 97:5440–5444
Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338:949–953
Ozawa Y (2010) Neurodegenerative disease: pieces of the Parkinson's puzzle. Nat Rev Neurosci 11:787
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V (2015) Alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522:340–344
Sulzer D (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci 30:244–250
Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S (2003) D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901
Walsh JG, Muruve DA, Power C (2014) Inflammasomes in the CNS. Nat Rev Neurosci 15:84–97
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD (2015) The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269
Zha QB, Wei HX, Li CG, Liang YD, Xu LH, Bai WJ, Pan H, He XH, Ouyang DY (2016) ATP-induced Inflammasome activation and Pyroptosis is regulated by AMP-activated protein kinase in macrophages. Front Immunol 7:597
Author information
Authors and Affiliations
Contributions
RG conceived the project, designed experiments and supervised the study. VD and EA performed experiments and data analysis with assistance from KZ and RG. RG and VD wrote the manuscript. All authors edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Deora, V., Albornoz, E.A., Zhu, K. et al. The Ketone Body β-Hydroxybutyrate Does Not Inhibit Synuclein Mediated Inflammasome Activation in Microglia. J Neuroimmune Pharmacol 12, 568–574 (2017). https://doi.org/10.1007/s11481-017-9754-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-017-9754-5