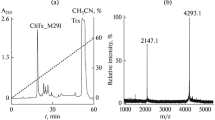
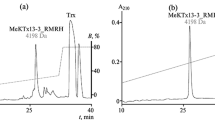
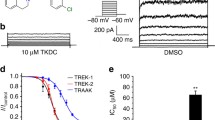
Abstract
Potassium voltage-gated Kv1.6 channel, which is distributed primarily in neurons of central and peripheral nervous systems, is of significant physiological importance. To date, several high-affinity Kv1.6-channel blockers are known, but the lack of selective ones among them hampers the studies of tissue localization and functioning of Kv1.6 channels. Here we present an approach to advanced understanding of interactions of peptide toxin blockers with a Kv1.6 pore. It combines molecular modeling studies and an application of a new bioengineering system based on a KcsA-Kv1.6 hybrid channel for the quantitative fluorescent analysis of blocker-channel interactions. Using this system we demonstrate that peptide toxins agitoxin 2, kaliotoxin1 and OSK1 have similar high affinity to the extracellular vestibule of the K+-conducting pore of Kv1.6, hetlaxin is a low-affinity ligand, whereas margatoxin and scyllatoxin do not bind to Kv1.6 pore. Binding of toxins to Kv1.6 pore has considerable inverse dependence on the ionic strength. Model structures of KcsA-Kv1.6 and Kv1.6 complexes with agitoxin 2, kaliotoxin 1 and OSK1 were obtained using homology modeling and molecular dynamics simulation. Interaction interfaces, which are formed by 15–19 toxin residues and 10 channel residues, are described and compared. Specific sites of Kv1.6 pore recognition are identified for targeting of peptide blockers. Analysis of interactions between agitoxin 2 derivatives with point mutations (S7K, S11G, L19S, R31G) and KcsA-Kv1.6 confirms reliability of the calculated complex structure.







Similar content being viewed by others
Abbreviations
- AgTx2:
-
agitoxin-2
- CLSM:
-
confocal laser scanning microscopy
- IPTG:
-
isopropyl β-D-1-thiogalactopyranoside
- KTx:
-
kaliotoxin 1
- Kv:
-
voltage-gated potassium channel
- MgTx:
-
margatoxin
- MD:
-
molecular dynamics
- OSK1:
-
a toxin from Orthochirus scrobiculosus scorpion venom
- R-AgTx2:
-
agitoxin-2 labeled with 5(6)-carboxytetramethylrhodamine N-succinimidyl ester
- RMSD:
-
root mean-square deviation
- RP:
-
reverse-phase
- TEA:
-
tetraethylammonium chloride
- TFA:
-
trifluoroacetic acid
- 4AP:
-
4-aminopyridine
References
Aiyar J, Withka JM, Rizzi JP, et al. (1995) Topology of the pore-region of a K+ channel revealed by the NMR-derived structures of scorpion toxins. Neuron 15:1169–1181
Aiyar J, Rizzi JP, Gutman GA, Chandy KG (1996) The signature sequence of voltage-gated potassium channels projects into the external vestibule. J Biol Chem 271:31013–31016. doi:10.1074/jbc.271.49.31013
Alessandri-Haber N, Alcaraz G, Deleuze C, et al. (2002) Molecular determinants of emerging excitability in rat embryonic motoneurons. J Physiol 541:25–39
Anh HN, Hoang VDM, Kudryashova KS, et al (2013) Hetlaxin, a new toxin from the Heterometrus laoticus scorpion venom, interacts with voltage-gated potassium channel Kv1.3. Dokl Biochem Biophys 449:109–111. doi:10.1134/S1607672913020142
Banerjee A, Lee A, Campbell E, MacKinnon R (2013) Structure of a pore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel. ElIfe 2:e00594. doi:10.7554/eLife.00594
Bergeron ZL, Bingham J-P (2012) Scorpion toxins specific for potassium (K+) channels: a historical overview of peptide bioengineering. Toxins (Basel) 4:1082–1119. doi:10.3390/toxins4111082
Brahmajothi MV, Morales MJ, Rasmusson RL, et al. (1997) Heterogeneity in K+ channel transcript expression detected in isolated ferret cardiac myocytes. Pacing Clin Electrophysiol 20:388–396
Chen PC, Kuyucak S (2009) Mechanism and energetics of charybdotoxin unbinding from a potassium channel from molecular dynamics simulations. Biophys J 96:2577–2588. doi:10.1016/j.bpj.2008.12.3952
Chen PC, Kuyucak S (2012) Developing a comparative docking protocol for the prediction of peptide selectivity profiles: investigation of potassium channel toxins. Toxins (Basel) 4:110–138. doi:10.3390/toxins4020110
Chung YH, Shin C, Kim MJ, et al. (2001) Immunohistochemical study on the distribution of six members of the Kv1 channel subunits in the rat cerebellum. Brain Res 895:173–177
Coetzee WA, Amarillo Y, Chiu J, et al. (1999) Molecular diversity of K+ channels. Ann N Y Acad Sci 868:233–285
Cui M, Shen J, Briggs JM, et al. (2002) Brownian dynamics simulations of the recognition of the scorpion toxin P05 with the small-conductance calcium-activated potassium channels. J Mol Biol 318:417–428. doi:10.1016/S0022-2836(02)00095-5
Davies AM, Batchelor TJP, Eardley I, Beech DJ (2002) Potassium channel KV alpha1 subunit expression and function in human detrusor muscle. J Urol 167:1881–1886
Dutertre S, Lewis RJ (2010) Use of venom peptides to probe ion channel structure and function. J Biol Chem 285:13315–13320. doi:10.1074/jbc.R109.076596
Eriksson MA, Roux B (2002) Modeling the structure of agitoxin in complex with the shaker K+ channel: a computational approach based on experimental distance. Biophys J 83:2595–2609. doi:10.1016/S0006-3495(02)75270-3
Gao Y, Garcia ML (2003) Interaction of agitoxin 2, charybdotoxin, and iberiotoxin with potassium channels: selectivity between voltage-gated and maxi-K channels. Proteins 52:146–154
Garcia ML, Garcia-Calvo M, Hidalgo P, et al. (1994) Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus Quinquestriatus Var. Hebraeus venom. Biochemistry 33:6834–6839
Gavrilovici C, Pollock E, Everest M, Poulter MO (2012) The loss of interneuron functional diversity in the piriform cortex after induction of experimental epilepsy. Neurobiol Dis 48:317–328. doi:10.1016/j.nbd.2012.07.002
Glazebrook PA, Ramirez AN, Schild JH, et al. (2002) Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol 541:467–482
Gonzalez C, Baez-Nieto D, Valencia I, et al. (2012) K+ channels: function-structural overview. Commun Phys 2:2087–2149. doi:10.1002/cphy.c110047
Gordon D, Chen R, Chung S-H (2013) Computational methods of studying the binding of toxins from venomous animals to biological ion channels: theory and applications. Physiol Rev 93:767–802. doi:10.1152/physrev.00035.2012
Gross A, Mackinnon R (1996) Agitoxin Footprinting the shaker Potassium Channel pore. Neuron 16:399–406
Hao J, Padilla F, Dandonneau M, et al. (2013) Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron 77:899–914. doi:10.1016/j.neuron.2012.12.035
Hayes KC (2004) The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev 10:295–316
Hidalgo P, Mackinnon R (1995) Revealing the architecture of a K+ channel pore through mutant cycles with a peptide inhibitor. Science 268:307–310
Hoang AN, Vo HDM, Vo NP, et al. (2014) Vietnamese Heterometrus laoticus scorpion venom: evidence for analgesic and anti-inflammatory activity and isolation of new polypeptide toxin acting on Kv1.3 potassium channel. Toxicon 77:40–48. doi:10.1016/j.toxicon.2013.10.027
Jang SH, Ryu PD, Lee SY (2011) Dendrotoxin-κ suppresses tumor growth induced by human lung adenocarcinoma A549 cells in nude mice. J Vet Sci 12:35–40
Jorgensen WL, Maxwell DS, Tirado-rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118:11225–11236
Jouirou B, Mosbah A, Visan V, et al. (2004) Cobatoxin 1 from Centruroides noxius scorpion venom: chemical synthesis, three-dimensional structure in solution, pharmacology and docking on K+ channels. Biochem J 377:37–49. doi:10.1042/BJ20030977
Kirsch GE, Shieh CC, Drewe JA, et al. (1993) Segmental exchanges define 4-aminopyridine binding and the inner mouth of K+ pores. Neuron 11:503–512
Kohl B, Rothenberg I, Ali SA, et al. (2015) Solid phase synthesis, NMR structure determination of α-KTx3.8, its in silico docking to Kv1.X potassium channels, and electrophysiological analysis provide insights into toxin-channel selectivity. Toxicon 101:70–78. doi:10.1016/j.toxicon.2015.04.018
Kudryashova KS, Nekrasova OV, Kuzmenkov AI, et al. (2013) Fluorescent system based on bacterial expression of hybrid KcsA channels designed for Kv1.3 ligand screening and study. Anal Bioanal Chem 405:2379–2389. doi:10.1007/s00216-012-6655-6
Kuzmenkov AI, Vassilevski AA, Kudryashova KS, et al. (2015) Variability of Potassium Channel blockers in Mesobuthus eupeus scorpion venom with focus on Kv1.1: AN INTEGRATED TRANSCRIPTOMIC AND PROTEOMIC STUDY. J Biol Chem 290:12195–121209. doi:10.1074/jbc.M115.637611
Legros C, Pollmann V, Knaus HG, et al. (2000) Generating a high affinity scorpion toxin receptor in KcsA-Kv1.3 chimeric potassium channels. J Biol Chem 275:16918–16924. doi:10.1074/jbc.275.22.16918
Legros C, Schulze C, Garcia ML, et al. (2002) Engineering-specific pharmacological binding sites for peptidyl inhibitors of potassium channels into KcsA. Biochemistry 41:15369–15375
Ling J, Yingliang W (2007) Molecular mechanism of the sea anemone toxin ShK recognizing the Kv1.3 channel explored by docking and molecular dynamic simulations. J Chem Inf Model 47:1967–1972. doi:10.1021/ci700178w
Lipkind GM, Fozzard HA (1997) A model of scorpion toxin binding to voltage-gated K+ channels. J Membr Biol 158:187–196. doi:10.1007/s002329900256
MacKinnon R, Cohen S, Kuo A, et al. (1998) Structural conservation in prokaryotic and eukaryotic potassium channels. Science 280:106–109
Matus-Leibovitch N, Vogel Z, Ezra-Macabee V, et al. (1996) Chronic morphine administration enhances the expression of Kv1.5 and Kv1.6 voltage-gated K+ channels in rat spinal cord. Brain Res Mol Brain Res 40:261–270
Mondal S, Babu RM, Bhavna R, Ramakumar S (2007) In silico detection of binding mode of J-superfamily conotoxin pl14a with Kv1.6 channel. In Silico Biol 7:175–186
Mouhat S, Visan V, Ananthakrishnan S, et al. (2005) K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem J 385:95–104. doi:10.1042/BJ20041379
Mouhat S, Teodorescu G, Homerick D, et al. (2006) Pharmacological profiling of Orthochirus scrobiculosus toxin 1 analogs with a trimmed N-terminal domain. Mol Pharmacol 69:354–362. doi:10.1124/mol.105.017210
Naranjo D, Miller C (1996) A strongly interacting pair of residues on the contact surface of charybdotoxin and a shaker K+ channel. Neuron 16:123–130. doi:10.1016/S0896-6273(00)80029-X
Nekrasova OV, Ignatova AA, Nazarova AI, et al. (2009a) Recombinant Kv channels at the membrane of Escherichia coli bind specifically agitoxin2. J NeuroImmune Pharmacol 4:83–91. doi:10.1007/s11481-008-9116-4
Nekrasova O, Tagway A, Ignatova A, et al. (2009b) Studying of membrane localization of recombinant potassium channels in E.Coli. Acta Nat 1:91–95
Novoseletsky VN, Volyntseva AD, Shaitan KV, et al. (2016) Modeling of the binding of peptide blockers to voltage-gated potassium channels: approaches and evidence. Acta Nat 8:35–46
Park WS, Firth AL, Han J, Ko EA (2010) Patho-, physiological roles of voltage-dependent K+ channels in pulmonary arterial smooth muscle cells. J Smooth Muscle Res 46:89–105
Pérez-Verdaguer M, Capera J, Serrano-Novillo C, et al. (2016) The voltage-gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opin Ther Targets 20:577–591. doi:10.1517/14728222.2016.1112792
Pyrkov T, Chugunov A, Krylov N, et al. (2009) PLATINUM: a web tool for analysis of hydrophobic/hydrophilic organization of biomolecular complexes. Bioinformatics 25:1201–1202
Ranganathan R, Lewis JH, Mackinnon R (1996) Spatial localization of the K+ channel selectivity filter by mutant cycle – based structure analysis. Neuron 16:131–139
Rashid MH, Kuyucak S (2012) Affinity and selectivity of ShK toxin for the Kv1 potassium channels from free energy simulations. J Phys Chem B 116:4812–4822. doi:10.1021/jp300639x
Rashid MH, Kuyucak S (2014) Free energy simulations of binding of HsTx1 toxin to Kv1 potassium channels: the basis of Kv1.3/Kv1.1 selectivity. J Phys Chem B 118:707–716. doi:10.1021/jp410950h
Sali A, Blundell T (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Schmidt K, Eulitz D, Veh RW, et al. (1999) Heterogeneous expression of voltage-gated potassium channels of the shaker family (Kv1) in oligodendrocyte progenitors. Brain Res 843:145–160
Shah NH, Aizenman E (2014) Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl Stroke Res 5:38–58. doi:10.1007/s12975-013-0297-7
Tian C, Zhu R, Zhu L, et al. (2014) Potassium channels: structures, diseases, and modulators. Chem Biol Drug Des 83:1–26. doi:10.1111/cbdd.12237
Wang X, Umetsu Y, Gao B, et al. (2015) Mesomartoxin , a new K v 1. 2-selective scorpion toxin interacting with the channel selectivity filter. Biochem Pharmacol 93:232–239. doi:10.1016/j.bcp.2014.12.002
Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics 54. doi:10.1002/cpbi.3
Wu Y, Cao Z, Yi H, et al. (2004) Simulation of the interaction between ScyTx and small conductance calcium-activated potassium channel by docking and MM-PBSA. Biophys J 87:105–112. doi:10.1529/biophysj.103.039156
Xiang Z (2006) Advances in homology protein structure modeling. Curr Protein Pept Sci 7:217–227
Yu K, Fu W, Liu H, et al. (2004) Computational simulations of interactions of scorpion toxins with the voltage-gated potassium Ion Channel. Biophys J 86:3542–3555. doi:10.1529/biophysj.103.039461
Yu L, Sun C, Song D, et al. (2005) Nuclear magnetic resonance structural studies of a potassium channel-charybdotoxin complex. Biochemistry 44:15834–15841. doi:10.1021/bi051656d
Acknowledgments
This work is supported by the Russian Science Foundation (grant no. 14-14-00239). LSM710 microscope was granted by the M.V. Lomonosov Moscow State University Program of Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 13167 kb)
Rights and permissions
About this article
Cite this article
Nekrasova, O.V., Volyntseva, A.D., Kudryashova, K.S. et al. Complexes of Peptide Blockers with Kv1.6 Pore Domain: Molecular Modeling and Studies with KcsA-Kv1.6 Channel. J Neuroimmune Pharmacol 12, 260–276 (2017). https://doi.org/10.1007/s11481-016-9710-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9710-9