Abstract
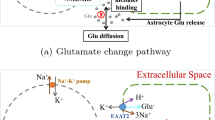
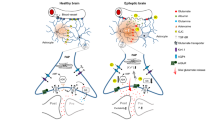
Gamma-aminobutyric acid (GABA) contributes substantially to neurocognitive function as an important inhibitory neurotransmitter in the human cerebral cortex. However, the pathophysiology of disorders such as epilepsy are not well understood, since GABA agonists are not quite effective in treating epilepsy. Knowledge of the mechanism of action of GABA would contribute to review previously proposed anti-epileptic processes by GABA agonists. In this study based on recent experiments on GABAergic astrocytes, we developed a modified GABAergic astrocyte model, and successfully simulated a long-lasting Ca2+ oscillation in astrocytes after 0.5-s stimulation of GABAergic transmission. We then incorporated this GABAergic astrocyte model into a classical Ullah-Schiff seizure model and surprisingly found that this GABAergic astrocyte model functions to hinder the anti-epileptic action of GABA agonists, thereby explaining their low efficiency in previous experiments. These results also update our knowledge of the mechanism of action of GABA and the effects of astrocytes on physiological and pathological functions of the brain.
Similar content being viewed by others
References
Purves D, Augustine G, Fitzpatrick D, et al. Neuroscience. 4th Ed. Sunderland, MA: Sinauer Associates, Incorporated, 2008
Chavas J, Marty A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J Neurosci, 2003, 23: 2019–2031
Roberts E, Fnnkel S. Gamma-aminobutyric acid in brain: Its formation from glutrrmic acid. J Biol Chem, 1950, 187: 55–63
Awapara J, Landua A J, Fuerst R, et al. Free γ-aminobutyric acid in brain. J Biol Chem, 1950, 187: 35–39
Florey E, McLennan H. The release of an inhibitory substance from mammalian brain, and its effect on peripheral synaptic transmission. J Physiol, 1955, 129: 384–392
Elliottand K A C, Gelder N M. Occlusion and metabolism of γ-aminobutyric acid by brain tissue. J Neurochem, 1958, 3: 28–40
Dichter M A. Physiological identification of GABA as the inhibitory transmitter for mammalian cortical neurons in cell culture. Brain Res, 1980, 190: 111–121
Bernardi G, Marciani M G, Stanzione P, et al. Evidence in favour of GABA as an inhibitory transmitter in the rat striatum. Adv Biochem Psychopharmacol, 1981, 30: 69–77
Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci, 2005, 28: 278–283
Zarrindast M R, Noorbakhshnia M, Motamedi F, et al. Effect of the GABAergic system on memory formation and state-dependent learning induced by morphine in rats. Pharmacology, 2006, 76: 93–100
Tellez R, Gómez-Viquez L, Liy-Salmeron G, et al. GABA, glutamate, dopamine and serotonin transporters expression on forgetting. Neurobiol Learning Memory, 2012, 98: 66–77
Gale K. GABA and epilepsy: Basic concepts from preclinical research. Epilepsia, 1992, 33 Suppl 5: S3–S12
Bradford H F. Glutamate, GABA and epilepsy. Prog Neurobiol, 1995, 47: 477–511
Brooks-Kayal A R, Shumate M D, Jin H, et al. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med, 1998, 4: 1166–1172
Kandel E R, Schwartz J H, Jessell T M, et al. Principles of Neural Science. New York: McGraw-Hill, 2000
Guo D, Li C. Stochastic resonance in Hodgkin-Huxley neuron induced by unreliable synaptic transmission. J Theor Biol, 2012, 308: 105–114
Uzuntarla M. Inverse stochastic resonance induced by synaptic background activity with unreliable synapses. Phys Lett A, 2013, 377: 2585–2589
Deng B, Wang J, Wei X. Effect of chemical synapse on vibrational resonance in coupled neurons. Chaos, 2009, 19: 013117
Perc M. Optimal spatial synchronization on scale-free networks via noisy chemical synapses. Biophysical Chem, 2009, 141: 175–179
Burić N, Todorović K, Vasović N. Synchronization of bursting neurons with delayed chemical synapses. Phys Rev E, 2008, 78: 036211
Zhao Z, Gu H. The influence of single neuron dynamics and network topology on time delay-induced multiple synchronous behaviors in inhibitory coupled network. Chaos Solitons Fractals, 2015, 80: 96–108
Wang Q Y, Lu Q S, Zheng Y H. Conduction delay-aided synchronization in two coupled chay neurons with inhibitory synapse. Acta Bioph Sin, 2005, 21: 449–456
Wang X J, Buzsák G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model, J Neurosci, 1996, 16: 6402–6413
Volk D W, Gonzalez-Burgos G, Lewis D A. l-Proline, GABA synthesis and gamma oscillations in schizophrenia. Trends Neurosci, 2016, 39: 797–798
Fábera P, Mareš P. Effect of GABA(B) receptor agonist SKF97541 on cortical and hippocampal epileptic afterdischarges. Physiol Res, 2014, 63: 529–534
Pedley T A, Horton R W, Meldrum B S. Electroencephalographic and behavioral effects of a GABA agonist (muscimol) on photosensitive epilepsy in the baboon, papio papio. Epilepsia, 1979, 20: 409–416
Nakata M, Mukawa J, Fromm G H. Why are most GABA agonists not effective antiepileptic drugs? Jpn J Psychiatry Neurol, 1991, 45: 391–394
Kocsis J D, Mattson R H. GABA levels in the brain: A target for new antiepileptic drugs. Neuroscientist, 1996, 2: 326–334
Mares P, Lindovský J, Slamberová R, et al. Effects of a GABA-B receptor agonist baclofen on cortical epileptic afterdischarges in rats. Epileptic Disord, 2007, 9 Suppl 1: S44–S51
Mares P, Tabashidze N. Contradictory effects of GABA-B receptor agonists on cortical epileptic afterdischarges in immature rats. Brain Res Bull, 2008, 75: 173–178
Fiacco T A, Agulhon C, McCarthy K D. Sorting out astrocyte physiology from pharmacology. Annu Rev Pharmacol Toxicol, 2009, 49: 151–174
Charles A C, Merrill J E, Dirksen E R, et al. Intercellular signaling in glial cells: Calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron, 1991, 6: 983–992
Pasti L, Volterra A, Pozzan T, et al. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci, 1997, 17: 7817–7830
Parpura V, Haydon P G. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci USA, 2000, 97: 8629–8634
Parri H R, Gould T M, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci, 2001, 4: 803–812
Araque A, Parpura V, Sanzgiri R P, et al. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci, 1999, 22: 208–215
Araque A, Carmignoto G, Haydon P G. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol, 2001, 63: 795–813
Perea G, Navarrete M, Araque A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci, 2009, 32: 421–431
Volman V, Ben-Jacob E, Levine H. The astrocyte as a gatekeeper of synaptic information transfer. Neural Comput, 2007, 19: 303–326
Tang J, Luo J M, Ma J. Information transmission in a neuron-astrocyte coupled model. PLoS ONE, 2013, 8: e80324
Li J J, Du M M, Wang R, et al. Astrocytic gliotransmitter: Diffusion dynamics and induction of information processing on tripartite synapses. Int J Bifurcation Chaos, 2016, 26: 1650138
Vélez-Fort M, Audinat E, Angulo M C. Central role of GABA in neuron- glia interactions. Neuroscientist, 2012, 18: 237–250
Losi G, Mariotti L, Carmignoto G. GABAergic interneuron to astrocyte signalling: A neglected form of cell communication in the brain. Philos Trans R Soc B-Biol Sci, 2014, 369: 20130609
Shigetomi E, Patel S, Khakh B S. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol, 2016, 26: 300–312
Bazargani N, Attwell D. Astrocyte calcium signaling: The third wave. Nat Neurosci, 2016, 19: 182–189
Mariotti L, Losi G, Sessolo M, et al. The inhibitory neurotransmitter GABA evokes long-lasting Ca2+ oscillations in cortical astrocytes. Glia, 2016, 64: 363–373
Pinsky P F, Rinzel J. Intrinsic and network rhythmogenesis in a reduced traub model for CA3 neurons. J Comput Neurosci, 1994, 1: 39–60
Amiri M, Bahrami F, Janahmadi M. Functional contributions of astrocytes in synchronization of a neuronal network model. J Theor Biol, 2012, 292: 60–70
Ullah G, Schiff S J. Assimilating seizure dynamics. PLoS Comput Biol, 2010, 6: e1000776
Nadkarni S, Jung P. Modeling synaptic transmission of the tripartite synapse. Phys Biol, 2007, 4: 1–9
Ullah G, Jung P, Cornell-Bell A H. Anti-phase calcium oscillations in astrocytes via inositol (1,4,5)-trisphosphate regeneration. Cell Calcium, 2006, 39: 197–208
Li J, Tang J, Ma J, et al. Dynamic transition of neuronal firing induced by abnormal astrocytic glutamate oscillation. Sci Rep, 2016, 6: 32343
Sahlender D A, Savtchouk I, Volterra A. What do we know about gliotransmitter release from astrocytes? Philos Trans R Soc B-Biol Sci, 2014, 369: 20130592
Guo D, Wu S, Chen M, et al. Regulation of irregular neuronal firing by autaptic transmission. Sci Rep, 2016, 6: 26096
Yilmaz E, Baysal V, Perc M, et al. Enhancement of pacemaker induced stochastic resonance by an autapse in a scale-free neuronal network. Sci China Tech Sci, 2016, 59: 364–370
Manning T J, Sontheimer H. Spontaneous intracellular calcium oscillations in cortical astrocytes from a patient with intractable childhood epilepsy (Rasmussen’s Encephalitis). Glia, 1997, 21: 332–337
Balázsi G, Cornell-Bell A H, Moss F. Increased phase synchronization of spontaneous calcium oscillations in epileptic human versus normal rat astrocyte cultures. Chaos, 2003, 13: 515–518
Kanemaru K, Okubo Y, Hirose K, et al. Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. J Neurosci, 2007, 27: 8957–8966
Bikson M, Hahn P J, Fox J E, et al. Depolarization block of neurons during maintenance of electrographic seizures. J Neurophysiol, 2003, 90: 2402–2408
Cressman Jr J R, Ullah G, Ziburkus J, et al. The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. J Comput Neurosci, 2009, 26: 159–170
Du M, Li J, Wang R, et al. The influence of potassium concentration on epileptic seizures in a coupled neuronal model in the hippocampus. Cogn Neurodyn, 2016, 10: 405–414
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, J., Xie, Y., Yu, Y. et al. A neglected GABAergic astrocyte: Calcium dynamics and involvement in seizure activity. Sci. China Technol. Sci. 60, 1003–1010 (2017). https://doi.org/10.1007/s11431-016-9056-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-016-9056-2