Abstract
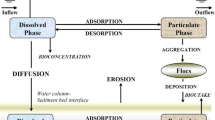
Based on the previous work on the transport-transformation of heavy metal pollutants in fluvial rivers, this paper presented the formulation of a two-dimensional model to describe heavy metal transport-transformation in fluvial rivers by considering basic principles of environmental chemistry, hydraulics, mechanics of sediment transport and recent developments along with three very simplified test cases. The model consists of water flow governing equations, sediment transport governing equations, transport-transformation equation of heavy metal pollutants, and convection-diffusion equations of adsorption-desorption kinetics of particulate heavy metal concentrations on suspended load, bed load and bed sediment. The heavy metal transport-transformation equation is basically a mass balance equation, which demonstrates how sediment transport affects transport-transformation of heavy metals in fluvial rivers. The convection-diffusion equations of adsorption-desorption kinetics of heavy metals, being an extension of batch reactor experimental results and a major advancement of the previous work, take both physical transport, i.e. convection and diffusion and chemical reactions, i.e. adsorption-desorption into account. Effects of sediment transport on heavy metal transport-transformation were clarified through three examples. Specifically, the transport-transformation of heavy metals in a steady, uniform and equilibrium sediment-laden flow was calculated by applying this model, and results were shown to be rational. Both theoretical analysis and numerical simulation indicated that the transport-transformation of heavy metals in sediment-laden flows with clay-enriched riverbed possesses not only the generality of common tracer pollutants, but also characteristics of transport-transformation induced by sediment motion. Future work will be conducted to present validation/application of the model with available data.
Similar content being viewed by others
Abbreviations
- x and y:
-
coordinates of two dimensions in plane
- dx and dy :
-
differential lengths in x and y directions
- h1, h2 and h3:
-
vertical lengths occupied by suspended load (flowing water), bed load (flowing water) and bed deformation
- h :
-
water depth (h 1 + h 2)
- c :
-
vertically averaged dissolved heavy metal concentration
- u and v:
-
vertically averaged flow velocities in x and y directions (flowing water and suspended load
- w x and w y :
-
vertically averaged velocities of bed load in x and y directions (flowing water and bed load)
- ss and sb:
-
suspended load concentration and bed load concentration
- Ns, Nb and Nm:
-
vertically averaged particulate concentrations of heavy metal on suspended load, bed load and sediment of the riverbed perimeter (or heavy metal contents sorbed by unit weights of suspended load, bed load and unit area of the riverbed sediment)
- Δh :
-
active thickness of bed sediment for adsorption-desorption
- ρ s :
-
specific weight of sediment particles
- p′:
-
the porosity of bed
- E x and E y :
-
turbulent diffusion coefficient components of flow
- E s x and E s y :
-
turbulent diffusion coefficient components of suspended load
- N :
-
particulate heavy metal concentration in batch reactor experiments at any time
- C :
-
dissolved heavy metal concentration in batch reactor experiments at any time
- k1, k2 and b:
-
coefficient of adsorption rate, coefficient of desorption rate and maximum adsorption capacity
- E Ns x and E Ns y :
-
turbulent diffusion coefficients of particulate heavy metal concentrations on suspended load in x and y directions
- k1b, k2b and bb:
-
coefficient of adsorption rate, coefficient of desorption rate, and maximum adsorption capacity for suspended load
- k1m, k2m and bm:
-
coefficient of adsorption rate, coefficient of desorption rate and maximum adsorption capacity for bed load
- k1m, k2m and bm:
-
coefficient of adsorption rate, coefficient of desorption rate and maximum sorption capacity for bed sediment
- z 0 :
-
bed elevation
- c 0 :
-
designated dissolved pollutant concentration
- δ(t):
-
δ function with time
- L :
-
length of the numerical flume
- s*:
-
suspended sediment-carrying capacity
- ks and ms:
-
coefficients in suspended sediment-carrying capacity formula
References
Hart B T. Water Quality Management—The Role of Particulate Matter in the Transport and Fate of Pollutants. Melbourne: Water Studies Center, Chisholm Institute of Technology, 1986. 10
Huang S L. The effect of sediment motion on transport and transformation of heavy metal pollutants (in Chines). Dissertation of Doctoral Degree. Beijing: Chinese Institute of Water Conservancy and Hydroelectric Power Research, 1993
Ellison M E, Brett M T. Particulate phosphorus bioavailability as a function of stream flow and land cover. Water Res, 2006, 40(6): 1258–1268
Huang S L, Wan Z H, Smith P. Numerical modeling of heavy metal pollutant transport-transformation in fluvial rivers: A review. Int J Sediment Res, 2007, 22(1): 16–26
Mossman D J, Schnoor J L, Stumm W. Predicting the effects of a pesticide release to the Rhine River. J WPCF, 1988, 60(10): 1806–1812
Allen-King R M, Grathwohl P, Ball W P. New modeling paradigms for the sorption of hydrophobic organic chemicals to heterogeneous carbonaceous matter in soils, sediments, and rocks. Adv Water Res, 2002, 25(8): 985–1016
Kim D M, Nakada N, Horiguchi T, et al. Numerical simulation of organic chemicals in a marine environment using a coupled 3D hydrodynamic and ecotoxicological model. Mar Pollut Bull, 2004, 48(7–8): 671–678
Koelmans A A, Heijde A V D, Knijff L M, et al. Integrated modeling of eutrophication and organic contaminant fate & effects in aquatic ecosystems. Water Res, 2001, 35(15): 3517–3536
Mahler B J, Perwonné J C, Lods G F, et al. Transport of free and particulate-associated bacteria in karst. J Hydrol, 2000, 238(3–4): 179–193
Khondaker A N. Modeling the fate of drilling waste in marine environment—an overview. Comput Geosci, 2000, 26(5): 531–540
Ziegler C K, Israelsson P H, Connolly J P. Modeling sediment transport dynamics in Thompson Island pool, upper Hudson River. Water Qual Ecosyst Model, 2000, 30(1): 193–222
Ji Z G, Hamrick J H, Pagenkopf J. Sediment and metals modeling in shallow river. J Environ Eng, 2002, 128(2): 105–119
James I D. Modeling pollution dispersion, the ecosystem and water quality in coastal waters: a review. Environ Model & Softw, 2002, 17(4): 363–385
Huang S L, Wan Z H, Smith P. Numerical modeling of heavy metal pollutant transport-transformation in fluvial rivers. J Hydraul Res, IAHR, 2007, 45(4): 451–461
Huang S L. Effect of using different sediment transport formulae and methods of computing Manning’s roughness coefficient on numerical modeling of sediment transport. J Hydraul Res, IAHR, 2007, 45(3): 347–356
Huang S L, Ng C O, Guo Q Z. Experimental investigation of the effect of flow turbulence and sediment transport patterns on the adsorption of cadmium ions onto sediment particles. J Environ Sci, 2007, 19(6): 696–703
Mckinley P J, Jenne E A. Exerimental investigation and review of the “solids concentration” effect in adsorption studies. Environ Sci Technol, 1991, 25(12): 2082–2087
Huang S L, Wan Z H. Present situation of heavy metal pollutant adsorption by sediment. Int J Sediment Res, 1995, 10(3): 69–81
Jain C K, Ram D. Adsorption of lead and zinc on bed sediments of the river Kali. Water Res, 1997, 31(1): 154–162
Trivedi P, Axe L. Modeling Cd and Zn sorption to hydrous metal oxides. Environ Sci Technol, 2000, 34(11): 2215–2223
O’Connor D J. Models of sorptive toxic substances in freshwater systems. I: Basic equations. J Environ Eng, ASCE, 1988, 114(3): 507–532
Huang S L. Adsorption of cadmium ions onto the Yellow River sediments. Water Qual Res J Canada, 2003, 38(2): 413–432
Huang S L. Investigation of cadmium desorption from different sized sediments. J Environ Eng, ASCE, 2003, 129(3): 241–247
IWRPR. Effects of Sediment from the Middle of the Yellow River on Heavy Metal Pollutant Transport-Transformation (in Chinese). Beijing: Water Resources of the Yellow River Protection and Research Institute, 1998. 56
Huang S L, Wan Z H. Concurrent sorption of heavy metal pollutants by sediment with different grain sizes. J Hydrodynam Ser B, 1997, 9(2): 1–12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, S. Equations and their physical interpretation in numerical modeling of heavy metals in fluvial rivers. Sci. China Technol. Sci. 53, 548–557 (2010). https://doi.org/10.1007/s11431-009-0389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-009-0389-5