Abstract
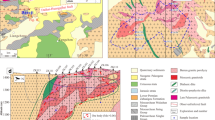
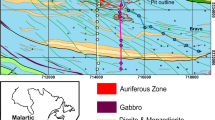
In this study, we performed an integrated investigation of K and Mg isotopes in hydrothermally altered rocks from the giant Dexing porphyry Cu deposit in China. Both the altered porphyry intrusion and the surrounding wall rocks exhibit large variations in K and Mg isotope compositions, with δ41K values ranging between −1.0296‰ and 0.38‰, and δ26Mg values ranging between −0.49‰ and 0.32‰. The δ41K and δ26Mg values of the majority of altered samples are higher than the isotopic baseline values for upper continental crust. We attribute the general increase in δ41K and δ26Mg in altered rocks to hydrothermal alteration, which caused preferential incorporation of heavy K and Mg isotopes in alteration products, particularly phyllosilicates. However, a few altered samples show anomalously low δ41K and δ26Mg values. The δ41K and δ26Mg values do not correlate with K and Mg concentrations, or mineralogy of altered samples. The variable K-Mg isotope data likely reflect fluids of different physical-chemical properties, or different isotopic compositions. Based on the combined K-Mg isotope data, at least three groups of hydrothermal fluids are distinguished from the Dexing porphyry deposit. Therefore, K-Mg isotopes are potentially a novel tracer for fingerprinting fluids in complex hydrothermal systems.
Similar content being viewed by others
References
Bailey S W. 1984. Classification and structures of the micas. Rev Mineral Geochem, 13: 1–12
Chen A A, Pappu R V. 2007. Quantitative characterization of ion pairing and cluster formation in strong 1:1 electrolytes. J Phys Chem B, 111: 6469–6478
Chen H, Tian Z, Tuller-Ross B, Korotev R L, Wang K. 2019. High-precision potassium isotopic analysis by MC-ICP-MS: An inter-laboratory comparison and refined K atomic weight. J Anal At Spectrom, 34: 160–171
Dauphas N, John S G, Rouxel O. 2017. Iron isotope systematics. Rev Mineral Geochem, 82: 415–510
Elderfield H, Schultz A. 1996. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu Rev Earth Planet Sci, 24: 191–224
Fennell C J, Bizjak A, Vlachy V, Dill K A. 2009. Ion pairing in molecular simulations of aqueous alkali halide solutions. J Phys Chem B, 113: 6782–6791
Gagnevin D, Boyce A J, Barrie C D, Menuge J F, Blakeman R J. 2012. Zn, Fe and S isotope fractionation in a large hydrothermal system. Geochim Cosmochim Acta, 88: 183–198
He M J, Liu X D, Lu X C, Wang R C. 2017. Molecular simulation study on K+-Cl− ion pair in geological fluids. Acta Geochim, 36: 1–8
Ho P C, Palmer D A. 1997. Ion association of dilute aqueous potassium chloride and potassium hydroxide solutions to 600°C and 300 MPa determined by electrical conductance measurements. Geochim Cosmochim Acta, 61: 3027–3040
Hu Y, Chen X Y, Xu Y K, Teng F Z. 2018. High-precision analysis of potassium isotopes by HR-MC-ICPMS. Chem Geol, 493: 100–108
Jin Z D. 1999. Geochemistry and evolution ore-forming fluids at Tongchang poprhyry copper deposit, Dexing county, Jiangxi province, Departaient of Earth Sciences (in Chinese). Dissertation for Doctoral Degree. Nanjing: Nanjing University. 114
Jin Z D, Zhu J C, Ji J F, Lu X W, Li F C. 2001. Ore-forming fluid constraints on illite crystallinity (IC) at Dexing porphyry copper deposit, Jiangxi Province. Sci China Ser-D Earth Sci, 44: 177–184
Johnson C M, Beard B L, Albarede F. 2004. Geochemistry of non-traditional stable isotopes. Mineralogical Society of America Geochemical Society
Li S L, Li W Q, Beard B L, Raymo M E, Wang X M, Chen Y, Chen J. 2019. K isotopes as a tracer for continental weathering and geological K cycling. Proc Natl Acad Sci USA, 116: 8740–8745
Li W Q, Beard B L, Li S L. 2016. Precise measurement ofstable potassium isotope ratios using a single focusing collision cell multi-collector ICP-MS. J Anal At Spectrom, 31: 1023–1029
Li W Q, Beard B L, Li C X, Johnson C M. 2014. Magnesium isotope fractionation between brucite [Mg(OH)2] and Mg aqueous species: Implications for silicate weathering and biogeochemical processes. Earth Planet Sci Lett, 394: 82–93
Li W Q, Jackson S E, Pearson N J, Graham S. 2010. Copper isotopic zonation in the Northparkes porphyry Cu-Au deposit, SE Australia. Geochim Cosmochim Acta, 74: 4078–4096
Li W Q, Johnson C M, Beard B L. 2012. U-Th-Pb isotope data indicate phanerozoic age for oxidation of the 3.4 Ga Apex Basalt. Earth Planet Sci Lett, 319–320: 197–206
Li W Q, Kwon K D, Li S L, Beard B L. 2017. Potassium isotope fractionation between K-salts and saturated aqueous solutions at room temperature: Laboratory experiments and theoretical calculations. Geochim Cosmochim Acta, 214: 1–13
Li W Q, Li S L, Beard B L. 2019. Geological cycling of potassium and the K isotopic response: Insights from loess and shales. Acta Geochim, 38: 508–516
Li X F, Sasaki M. 2007. Hydrothermal alteration and mineralization of middle Jurassic dexing porphyry Cu-Mo deposit, Southeast China. Resour Geol, 57: 409–426
Mathur R, Munk L, Nguyen M, Gregory M, Annell H, Lang J. 2013. Modern and paleofluid pathways revealed by Cu isotope compositions in surface waters and ores of the Pebble porphyry Cu-Au-Mo deposit, Alaska. Econ Geol, 108: 529–541
Morgan L E, Santiago Ramos D P, Davidheiser-Kroll B, Faithfull J, Lloyd N S, Ellam R M, Higgins J A. 2018. High-precision 41K/39K measurements by MC-ICP-MS indicate terrestrial variability of δ 41K. J Anal At Spectrom, 33: 175–186
Pan X F, Song Y C, Li Z Q, Hu B G, Zhu X Y, Wang Z K, Yang D, Zhang T F, Li Y. 2012. Restriction of H-O iostopes for alteration and mineralization ssytem of Tongchang Cu (-Mo-Au) porphyric deposit, Jiangxi Province (in Chinese). Mineral Deposits, 31: 850–860
Parendo C A, Jacobsen S B, Wang K. 2017. K isotopes as a tracer of seafloor hydrothermal alteration. Proc Natl Acad Sci USA, 114: 1827–1831
Pirajno F. 2009. Hydrothermal Processes and Mineral Systems. Springer Netherlands. 1241
Putnis A. 2009. Mineral replacement reactions. Rev Mineral Geochem, 70: 87–124
Reed M H. 1997. Hydrothermal alteration and its relationship to ore fluid composition. In: Barnes H L, ed. Geochemistry of Hydrothermal Ore Deposits. 3rd ed. New York: Wiley. 303–366
Ryu J S, Vigier N, Decarreau A, Lee S W, Lee K S, Song H, Petit S. 2016. Experimental investigation of Mg isotope fractionation during mineral dissolution and clay formation. Chem Geol, 445: 135–145
Santiago-Ramos D P, Morgan L E, Lloyd N S, Higgins J A. 2018. Reverse weathering in marine sediments and the geochemical cycle of potassium in seawater: Insights from the K isotopic composition (41K/39K) of deep-sea pore-fluids. Geochim Cosmochim Acta, 236: 99–120
Schauble E A. 2004. Applying stable isotope fractionation theory to new systems. Rev Mineral Geochem, 55: 65–111
Schott J, Mavromatis V, Fujii T, Pearce C R, Oelkers E H. 2016. The control of carbonate mineral Mg isotope composition by aqueous speciation: Theoretical and experimental modeling. Chem Geol, 445: 120–134
Seedorff E, Dilles J H, Proffett J M, Einaudi M T, Zurcher L, Stavast W J A, Johnson D A, Darton M D. 2005. Porphyry deposits: Characteristics and origin of hypogene features. Econ Geol 100th Anniversary Volume. 251–298
Seo J H, Lee S K, Lee I. 2007. Quantum chemical calculations of equilibrium copper (I) isotope fractionations in ore-forming fluids. Chem Geol, 243: 225–237
Sillitoe R H. 2010. Porphyry copper systems. Econ Geol, 105: 3–41
Teng F Z. 2017. Magnesium isotope geochemistry. Rev Mineral Geochem, 82: 219–287
Teng F Z, Dauphas N, Watkins J M. 2017. Non-traditional stable isotopes: Retrospective and prospective. Rev Mineral Geochem, 82: 1–26
Teng F Z, Li W Y, Ke S, Marty B, Dauphas N, Huang S C, Wu F Y, Pourmand A. 2010. Magnesium isotopic composition of the Earth and chondrites. Geochim Cosmochim Acta, 74: 4150–4166
Teng F Z, McDonough W F, Rudnick R L, Walker R J. 2006. Diffusion-driven extreme lithium isotopic fractionation in country rocks ofthe Tin Mountain pegmatite. Earth Planet Sci Lett, 243: 701–710
Wang G G, Ni P, Yao J, Wang X L, Zhao K D, Zhu R Z, Xu Y F, Pan J Y, Li L, Zhang Y H. 2015. The link between subduction-modified lithosphere and the giant Dexing porphyry copper deposit, South China: Constraints from high-Mg adakitic rocks. Ore Geol Rev, 67: 109–126
Wang K, Jacobsen S B. 2016. An estimate of the Bulk Silicate Earth potassium isotopic composition based on MC-ICPMS measurements of basalts. Geochim Cosmochim Acta, 178: 223–232
Wang Y, Zhu X K, Cheng Y B. 2015. Fe isotope behaviours during sulfide-dominated skarn-type mineralisation. J Asian Earth Sci, 103: 374–392
Wang Y, Zhu X K, Mao J W, Li Z H, Cheng Y B. 2011. Iron isotope fractionation during skarn-type metallogeny: A case study of Xinqiao Cu-S-Fe-Au deposit in the Middle-Lower Yangtze valley. Ore Geol Rev, 43: 194–202
Weiss Z, Wiewiora A. 1986. Polytypism of micas. III. X-ray diffraction identification. Clays Clay Miner, 34: 53–68
Xu Y K, Hu Y, Chen X Y, Huang T Y, Sletten R S, Zhu D, Teng F Z. 2019. Potassium isotopic compositions of international geological reference materials. Chem Geol, 513: 101–107
Yao J M, Mathur R, Sun W D, Song W L, Chen H Y, Mutti L, Xiang X K, Luo X H. 2016. Fractionation of Cu and Mo isotopes caused by vapor-liquid partitioning, evidence from the Dahutang W-Cu-Mo ore field. Geochem Geophys Geosyst, 17: 1725–1739
Zhu X, Huang C K, Rui Z Y, Zhou Y H, Zhu X J, Hu C S, Mei Z K. 1983. Dexing Porphyry Copper Deposit (in Chinese). Beijing: Geological Publishing House
Zhu X K, O’Nions R K, Guo Y, Belshaw N S, Rickard D. 2000. Determination of natural Cu-isotope variation by plasma-source mass spectrometry: Implications for use as geochemical tracers. Chem Geol, 163: 139–149
Acknowledgements
Brian Beard helped in mass spectrometry for K isotope analysis. This manuscript benefited from constructive comments from two anonymous reviewers, as well as editorial handling by Prof. Fangzhen Teng. This study was supported by the National Key R & D Program of China (Grant No. 2018YFC0604106) and the National Natural Science Foundation of China (Grant Nos. 41622301, 41873004).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, W., Zhao, S., Wang, X. et al. Fingerprinting hydrothermal fluids in porphyry Cu deposits using K and Mg isotopes. Sci. China Earth Sci. 63, 108–120 (2020). https://doi.org/10.1007/s11430-018-9387-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11430-018-9387-2