Abstract
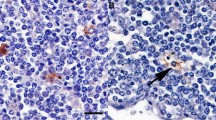
To obtain high titer monoclonal antibodies (McAbs) which can react with mammalian prion protein (PrP), Balb/C mice were immunized with bovine (Bo) PrP peptide (BoPrP 209–228 aa) coupled to keyhole limpet hemocyanin (KLH). The hybridoma cell lines secreting monoclonal antibodies against the peptide were established by cell fusion and cloning. The obtained McAbs were applied to detect recombinant human, bovine and hamster PrP, cellular prion protein (PrPc) in normal bovine brain and pathogenic scrapie prion protein (PrPSc) accumulated in the medulla oblongata of bovine spongiform encephalopathy(BSE)specimen with Western blot and immunohistochemical detection, respectively. The current procedure might offer a simple, feasible method to raise high titer antibodies for studying biological features of PrP in mammals, as well as detection of transmissible spongiform encephalopathy (TSE) and diagnosis of BSE, in particular.
Similar content being viewed by others
References
Harman J L, Silva C J. Bovine spongiform encephalopathy. J Am Vet Med Assoc. 2009, 234: 59–72 19119967, 10.2460/javma.234.1.59
Rezaei H. Prion protein oligomerization. Curr Alzheimer Res, 2008, 5: 572–578 19075584, 10.2174/156720508786898497, 1:CAS:528:DC%2BD1cXhsVKgsb3O
Llewelyn C A, Hewitt P E, Knight R S, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004, 363: 417–421 14962520, 10.1016/S0140-6736(04)15486-X, 1:STN:280:DC%2BD2c%2FnsVWhuw%3D%3D
Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell, 2004,116: 313–327 14744440, 10.1016/S0092-8674(03)01031-6, 1:CAS:528:DC%2BD2cXhtVals7g%3D
Kübler E, Oesch B, Raeber A J. Diagnosis of prion diseases. Br Med Bull, 2003, 66: 267–279 14522864, 10.1093/bmb/66.1.267
Wopfner F, Weidenhöfer G, Schneider R, et al. Analysis of 27 mammalian and 9 avian PrPs reveals high conservation of flexible regions of the prion protein. J Mol Biol, 1999, 289: 1163–1178 10373359, 10.1006/jmbi.1999.2831, 1:CAS:528:DyaK1MXjvVCiur8%3D
Hosokawa T, Tsuchiya K, Sato I, et al. A monoclonal antibody (1D12) defines novel distribution patterns of prion protein (PrP) as granules in nucleus. Biochem Biophys Res Commun, 2008, 366: 657–663 18068119, 10.1016/j.bbrc.2007.11.163, 1:CAS:528:DC%2BD1cXivVehtQ%3D%3D
Khalili-Shirazi A, Kaisar M, Mallinson G, et al. Beta-PrP form of human prion protein stimulates production of monoclonal antibodies to epitope 91–110 that recognize native PrPSc. Biochim Biophys Acta, 2007, 1774: 1438–1450 17936697, 1:CAS:528:DC%2BD2sXhtlGjurvF
Korth C, Stierli B, Streit P, et al. Prion(PrPSc)-Specific epitope defined a monoclonal antibody. Nature, 1997, 390: 74–77 9363892, 10.1038/36337, 1:CAS:528:DyaK2sXnt1Crtr8%3D
White A R, Enever P, Tayebi M, et al. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature, 2003, 422: 80–83 12621436, 10.1038/nature01457, 1:CAS:528:DC%2BD3sXhs1yitL4%3D
Gilch S, Wopfner F, Renner-Müller I, et al. Polyclonal anti-PrP auto-antibodies induced with dimeric PrP interfere efficiently with PrPSc propagation in prion-infected cells. J Biol Chem, 2003, 278: 18524–18531 12637572, 10.1074/jbc.M210723200, 1:CAS:528:DC%2BD3sXjs1Kht78%3D
Zhao L, Wang J W, JI R et al. Gene cloning and expression of hamster, human and bovine prion protein PrPc in Escherichia coli. J Shandong Univ (Health Sci), 2004, 42: 249–251 1:CAS:528:DC%2BD2MXlvFGmtbY%3D
Bundle D R, Gidney M A, Kassam N, et al. Hybridomas specific for carbohydrates; synthetic human blood group antigens for the production, selection, and characterization of monoclonal typing reagents. J Immunol, 1982, 129: 678–682 6177774, 1:CAS:528:DyaL38XkvFShsrs%3D
Harmeyer S, Pfaff E, Groschup, M H, et al. Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J Gen Virol, 1998, 79: 937–945 9568991, 1:CAS:528:DyaK1cXit1agt7g%3D
O’Rourke K I, Baszler T V, Miller J M, et al. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol, 1998, 36: 1750–1755 9620413
Schaller O, Fatzer R, Stack M, et al. Validation of a western immunoblotting procedure for bovine PrP(Sc) detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE). Acta Neuropathol, 1999, 98: 437–443 10541864, 10.1007/s004010051106, 1:CAS:528:DyaK1MXmtFOjtbw%3D
JMartucci F, Acutis P, Mazza M, et al. Detection of typical and atypical bovine spongiform encephalopathy and scrapie prion strains by prion protein motif-grafted antibodies. Gen Virol, 2009, 90: 1048–1053 10.1099/vir.2008.007120-0
Salguero F J, Díaz-San S F, Brun A, et al. Comparison of three monoclonal antibodies for use in immunohistochemical detection of bovine spongiform encephalopathy protease-resistant prion protein. J Vet Diagn Invest, 2006, 18: 106–109 16566267
Fujii F, Horiuchi M, Ueno M, et al. Detection of prion protein immune complex for bovine spongiform encephalopathy diagnosis using fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy. Anal Biochem, 2007, 370: 131–141 17825783, 10.1016/j.ab.2007.07.018, 1:CAS:528:DC%2BD2sXhtFSls7jI
Scott M R, Butler D A, Bredesen D E, et al. Prion protein gene expression in cultured cells, Protein Eng, 1988, 2: 69–76 2908139, 10.1093/protein/2.1.69, 1:CAS:528:DyaL1cXksV2hu7g%3D
Caughey B R, Race R E, Vogel M, et al. In vitro expression in eukaryotic cells of a prion protein gene cloned from scrapie-infected mouse brain. Prol Natl Acad Sci USA, 1988, 85: 4657–4661 10.1073/pnas.85.13.4657, 1:CAS:528:DyaL1cXkvVOiu70%3D
Rogers M, Serban D, Gyuris T, et al. Epitope mapping of the Syrian hamster prion protein utilizing chimeric and mutant genes in a vaccinia virus expression system. J Immunol, 1991, 147: 3568–3574 1719082, 1:CAS:528:DyaK38Xls1yltA%3D%3D
Weiss S, Famulok M, Edenhofer F, et al. Overexpression of active Syrian gold hamster prion protein PrPc as a glutathione-S-transferase fusion in heterologous systems. J Virol, 1995, 69: 4776–4783 7609044, 1:CAS:528:DyaK2MXmvFWgsrg%3D
Hanan E, Priola S A, Solomon B. Antiaggregating antibody raised against human PrP 106–126 recognizes pathological and normal isoforms of the whole prion protein. Cell Mol Neurobiol, 2001, 21: 693–703 12043842, 10.1023/A:1015199904354, 1:CAS:528:DC%2BD38XkslSktLo%3D
Takahashi H, Takahashi R H, Hasegawa H, et al. Characterization of antibodies raised against bovine-PrP-peptides. J Neurovirol, 1999, 5: 300–307 10414520, 10.3109/13550289909015816, 1:CAS:528:DyaK1MXkslelur4%3D
Schwarz A, Kratke O, Burwinkel M, et al. Immunization with a synthetic prion protein-derived peptide prolongs survival times of mice orally exposed to the scrapie agent. Neurosci Lett, 2003, 350: 187–189 14550926, 10.1016/S0304-3940(03)00907-8, 1:CAS:528:DC%2BD3sXnvVOjsbc%3D
Cernilec M, Vranac T, Hafner-Bratkovic I, et al. Identification of an epitope on the recombinant bovine PrP that is able to elicit a prominent immune response in wild-type mice. Immunol Lett, 2007, 113: 29–39 17884181, 10.1016/j.imlet.2007.07.012, 1:CAS:528:DC%2BD2sXhtFSnu73E
Wang J W, Wang D B, Luo X Y, et al. Preparation and characterization of monoclonal antibodies to tetrodotoxin. J Hyg Res, 1996, 25: 168–171
Matsuda H, Mitsude H, Nakamura N, et al. A chicken monoclonal antibody with specificity for the N-terminal of human prion protein. FEMS Immunol Med Microbiol, 1999, 23: 189–194 10219590, 10.1111/j.1574-695X.1999.tb01238.x, 1:CAS:528:DyaK1MXhvFCrsLg%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., Hou, X., Ji, R. et al. Establishment of bovine prion peptide-based monoclonal antibodies for identifying bovine prion. SCI CHINA SER C 52, 754–760 (2009). https://doi.org/10.1007/s11427-009-0100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-009-0100-z