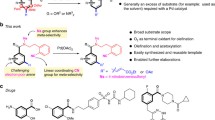
Abstract
The installation of azoles via C–H/N–H cross-coupling is significantly underdeveloped, particularly in benzylic C–H azolation due to the requirement for external chemical oxidants and the challenge in controlling the site- and chemo-selectivity. Herein, a late-stage azolation of benzylic C‒H bonds enabled by electrooxidation is described, which proceeds in an undivided cell under mild, catalyst- and chemical-oxidant-free reaction conditions. The strategy empowers the C‒H azolation on primary, secondary, and even challenging tertiary benzylic positions selectively. The remarkable synthetic utility of our approach is highlighted by its easy scalability without overoxidation of products and ample scope with valuable functional groups. The approach can be directly used to install benzyl and azole motifs on highly functionalized drug molecules.

Similar content being viewed by others
References
Schmidt B, Schieffer B. J Med Chem, 2003, 46: 2261–2270
Breschi MC, Calderone V, Digiacomo M, Martelli A, Martinotti E, Minutolo F, Rapposelli S, Balsamo A. J Med Chem, 2004, 47: 5597–5600
Zhang HZ, Gan LL, Wang H, Zhou CH. Mini-Rev Med Chem, 2017, 17: 122–166
Das P, Delost MD, Qureshi MH, Smith DT, Njardarson JT. J Med Chem, 2019, 62: 4265–4311
Al-Azmi A, George P, El-Dusouqui OME. J Heterocycl Chem, 2007, 44: 515–520
Vitaku E, Smith DT, Njardarson JT. J Med Chem, 2014, 57: 10257–10274
Kong D, Moon PJ, Bsharat O, Lundgren RJ. Angew Chem Int Ed, 2020, 59: 1313–1319
Fan X, Lei T, Liu Z, Yang XL, Cheng YY, Liang G, Chen B, Tung CH, Wu LZ. Eur J Org Chem, 2020, 2020: 1551–1558
Stivanin ML, Fernandes AAG, Silva AF, Okada Jr CY, Jurberg ID. Adv Synth Catal, 2020, 362: 1106–1111
Wang K, Chen P, Ji D, Zhang X, Xu G, Sun J. Angew Chem Int Ed, 2018, 57: 12489–12493
Sun HL, Yang F, Ye WT, Wang JJ, Zhu R. ACS Catal, 2020, 10: 4983–4989
Yang Y, Yu Y, Wang Y, Zhang Q, Li D. Tetrahedron, 2018, 74: 1085–1091
Yamamoto C, Takamatsu K, Hirano K, Miura M. J Org Chem, 2016, 81: 7675–7684
Ye L, Tian Y, Meng X, Gu Q, Liu X. Angew Chem Int Ed, 2020, 59: 1129–1133
Prier CK, Zhang RK, Buller AR, Brinkmann-Chen S, Arnold FH. Nat Chem, 2017, 9: 629–634
Clark JR, Feng K, Sookezian A, White MC. Nat Chem, 2018, 10: 583–591
Song C, Dong X, Yi H, Chiang CW, Lei A. ACS Catal, 2018, 8: 2195–2199
Wang X, Li C, Zhang Y, Zhang B, Sun K. Org Biomol Chem, 2019, 17: 8364–8368
Pandey G, Laha R, Singh D. J Org Chem, 2016, 81: 7161–7171
Xue Q, Xie J, Li H, Cheng Y, Zhu C. Chem Commun, 2013, 49: 3700–3702
Xia Q, Chen W, Qiu H. J Org Chem, 2011, 76: 7577–7582
For recent reviews on organic electrosynthesis: (a) Meyer TH, Finger LH, Gandeepan P, Ackermann L. Trends Chem, 2019, 1: 63–76
Waldvogel SR, Lips S, Selt M, Riehl B, Kampf CJ. Chem Rev, 2018, 118: 6706–6765
Tang S, Liu Y, Lei A. Chem, 2018, 4: 27–45
Hou ZW, Mao ZY, Xu HC. Synlett, 2017, 28: 1867–1872
Cardoso DSP, Šljukić B, Santos DMF, Sequeira CAC. Org Process Res Dev, 2017, 21: 1213–1226
Horn EJ, Rosen BR, Baran PS. ACS Cent Sci, 2016, 2: 302–308
Francke R, Little RD. Chem Soc Rev, 2014, 43: 2492–2521
Francke R. Beilstein J Org Chem, 2014, 10: 2858–2873
Yoshida J, Kataoka K, Horcajada R, Nagaki A. Chem Rev, 2008, 108: 2265–2299
Jutand A. Chem Rev, 2008, 108: 2300–2347
Minteer SD, Baran P. Acc Chem Res, 2020, 53: 545–546
Kingston C, Palkowitz MD, Takahira Y, Vantourout JC, Peters BK, Kawamata Y, Baran PS. Acc Chem Res, 2020, 53: 72–83
Röckl JL, Pollok D, Franke R, Waldvogel SR. Acc Chem Res, 2020, 53: 45–61
Siu JC, Fu N, Lin S. Acc Chem Res, 2020, 53: 547–560
Jiao KJ, Xing YK, Yang QL, Qiu H, Mei TS. Acc Chem Res, 2020, 53: 300–310
Leech MC, Lam K. Acc Chem Res, 2020, 53: 121–134
Yamamoto K, Kuriyama M, Onomura O. Acc Chem Res, 2020, 53: 105–120
Ackermann L. Acc Chem Res, 2020, 53: 84–104
Xiong P, Xu HC. Acc Chem Res, 2019, 52: 3339–3350
Meyer TH, Choi I, Tian C, Ackermann L. Chem, 2020, 6: 2484–2496
Zhang S, Samanta RC, Del Vecchio A, Ackermann L. Chem Eur J, 2020, 26: 10936–10947
Wang H, Gao X, Lv Z, Abdelilah T, Lei A. Chem Rev, 2019, 119: 6769–6787
Yuan Y, Lei A. Acc Chem Res, 2019, 52: 3309–3324
Feng P, Ma G, Chen X, Wu X, Lin L, Liu P, Chen T. Angew Chem Int Ed, 2019, 58: 8400–8404
Qiu Y, Struwe J, Meyer TH, Oliveira JCA, Ackermann L. Chem Eur J, 2018, 24: 12784–12789
Petrosyan VA, Burasov AV, Vakhotina TS. Russ Chem Bull, 2005, 54: 1197–1202
Hu K, Niyazymbetov ME, Evans DH. Tetrahedron Lett, 1995, 36: 7027–7030
de Robillard G, Makni O, Cattey H, Andrieu J, Devillers CH. Green Chem, 2015, 17: 4669–4679
Wan Z, Wang D, Yang Z, Zhang H, Wang S, Lei A. Green Chem, 2020, 22: 3742–3747
Wu J, Zhou Y, Zhou Y, Chiang CW, Lei A. ACS Catal, 2017, 7: 8320–8323
Shao X, Tian L, Wang Y. Eur J Org Chem, 2019, 2019(25): 4089–4094
Yang YZ, Song RJ, Li JH. Org Lett, 2019, 21: 3228–3231
Zeng C, Zhang N, Lam CM, Little RD. Org Lett, 2012, 14: 1314–1317
Xiong P, Zhao HB, Fan XT, Jie LH, Long H, Xu P, Liu ZJ, Wu ZJ, Cheng J, Xu HC. Nat Commun, 2020, 11: 2706–2714
Morofuji T, Shimizu A, Yoshida J. J Am Chem Soc, 2014, 136: 4496–4499
Lee BJ, DeGlopper KS, Yoon TP. Angew Chem Int Ed, 2020, 59: 197–202
Wang H, Liang K, Xiong W, Samanta S, Li W, Lei A. Sci Adv, 2020, 6: eaaz0590
Baciocchi E, Bietti M, Lanzalunga O. Acc Chem Res, 2000, 33: 243–251
Xu Z, Li Y, Mo G, Zheng Y, Zeng S, Sun PH, Ruan Z. Org Lett, 2020, 22: 4016–4020
Xu Z, Huang Z, Li Y, Kuniyil R, Zhang C, Ackermann L, Ruan Z. Green Chem, 2020, 22: 1099–1104
Ruan Z, Huang Z, Xu Z, Mo G, Tian X, Yu XY, Ackermann L. Org Lett, 2019, 21: 1237–1240
Li Y, Huang Z, Mo G, Jiang W, Zheng C, Feng P, Ruan Z. Chin J Chem, 2020, https://doi.org/10.1002/cjoc.202000586
When we are preparing the manuscript, a similar elegant work was reported by Xu, see: (a) Hou Z, Liu D, Xiong P, Lai X, Song J, Xu H. Angew Chem Int Ed, 2021, 60: 2943–2947
The methodology was mainly focused on amination of the secondary benzylic positions and less electronic benzylic substrates. Herein, our work mainly addressed the amination of electron-rich substrates with primary, secondary and tertiary benzylic positions The methodology was mainly focused on amination of the secondary benzylic positions and less electronic benzylic substrates. Herein, our work mainly addressed the amination of electron-rich substrates with primary, secondary and tertiary benzylic position
Garrett C, Prasad K. Adv Synthesis Catal, 2004, 346: 889–900
Caron S, Dugger RW, Ruggeri SG, Ragan JA, Ripin DHB. Chem Rev, 2006, 106: 2943–2989
The anodic oxidation of 1a was mainly affected by the specific electrode area and current density, see: Pletcher D. Industrial Electrochemistry. London, New York: Chapmann and Hall. 2ed. 1990. 79–90
Herein, the electrolyte of Et4NClO4 was employed for the CV test, because the CV of 2a was interfered by the oxidation of nBu4NHSO4
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21901052, 81872759), the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), the Guangzhou Education Bureau University Scientific Research Project (201831845) and the Guangdong Basic and Applied Basic Research Foundation (2020A1515010722).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Supporting Information
Rights and permissions
About this article
Cite this article
Ruan, Z., Huang, Z., Xu, Z. et al. Late-stage azolation of benzylic C‒H bonds enabled by electrooxidation. Sci. China Chem. 64, 800–807 (2021). https://doi.org/10.1007/s11426-020-9938-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-020-9938-9