Abstract
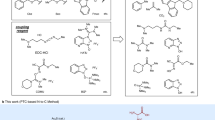
Cyclotides constitute a fascinating family of circular proteins containing ca. 30 amino acid residues. They have a unique cyclic cysteine knot topology and exhibit remarkable thermal, chemical and enzymatic stabilities. These characteristics enable them to have a range of biological activities and promising pharmaceutical and agricultural applications. Here, we present a practical strategy for the chemical synthesis of cyclotides through the intramolecular ligation of fully unprotected peptide O-esters. This strategy involves the mild Fmoc solid-phase peptide synthesis of the peptide O-ester backbone, the head-to-tail cyclization of the cyclotide backbone by native chemical ligation, and the oxidative refolding to yield the natural knot protein. The simplicity and high efficiency of the strategy can be employed in the synthesis of artificial cyclotides for pharmaceutical applications.
Similar content being viewed by others
References
Craik DJ, Daly NL, Mulvenna J, Plan MR, Trabi M. Discovery, structure and biological activities of the cyclotides. Curr Protein Pept Sci, 2004, 5: 297–315
Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol, 1999, 294: 1327–1336
Craik DJ, Conibear AC. The chemistry of cyclotides. J Org Chem, 2011, 76: 4805–4817
Ireland DC, Wang CK, Wilson JA, Gustafson KR, and Craik DJ. Cyclotides as natural anti-HIV agents. Biopolymers, 2008, 90: 51–60
Rosengren KJ, Goransson U, Otvos L, Craik D. Cyclization of pyrrhocoricin retains structural elements crucial for the antimicrobial activity of the native peptide. Biopolymers, 2004, 76: 446–458
Hernandez JF, Gagnon J, Chiche L, Nguyen TM, Andrieu JP, Heitz A, Hong TT, Pham TTC, Nguyen DL. Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry, 2000, 39: 5722–5730
Kisangau DP, Hosea KM, Lyaruu HVM, Joseph C, Mbwambo ZH, Masimba PJ, Gwandu CB, Bruno LN, Devkota KP, Sewald N. Screening of traditionally used Tanzanian medicinal plants for antifungal activity. Pharm Biol, 2009, 47: 708–716
Daly NL, Craik DJ. Design and therapeutic applications of cyclotides. Future Med Chem, 2009, 1: 1613–1622
Craik DJ, Daly NL. Bioactive cystine knot proteins. Cur Opin Chem Biol, 2011, 15: 362–368
Tam JP, Lu YA. Synthesis of large cyclic cystine-knot peptide by orthogonal coupling strategy using unprotected peptide precursor. Tetrahedron Lett, 1997, 38: 5599–5602
Tam JP, Lu YA, Yu QT. Thia zip reaction for synthesis of large cyclic peptides: Mechanisms and applications. J Am Chem Soc, 1999, 121: 4316–4324
Tam JP, Lu YA, Yang JL, Chiu KW. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystineknot disulfides. Proc Natl Acad Sci USA, 1999, 96: 8913–8918
Craik DJ, Daly NL, Love S, Alewood PF. Chemical synthesis and folding pathways of large cyclic polypeptide: Studies of the cystine knot polypeptide kalata B1. Biochemistry, 1999, 38: 10606–10614
Leatherbarrow RJ, Thongyoo P, Tate EW. Total synthesis of the macrocyclic cysteine knot microprotein MCoTI-II. Chem Commun, 2006, 2848–2850
Goransson U, Aboye TL, Clark RJ, Craik DJ. Ultra-stable peptide scaffolds for protein engineering — Synthesis and folding of the circular cystine knotted cyclotide cycloviolacin O2. ChemBioChem, 2008, 9: 103–113
Goransson U, Park S, Gunasekera S, Aboye TL. An Efficient Approach for the total synthesis of cyclotides by microwave assisted Fmoc-SPPS. Int J Pept Res Ther, 2010, 16: 167–176
Camarero JA, Muir TW. Chemoselective backbone cyclization of unprotected peptides. Chem Commun, 1997, 1369–1370
Schnolzer M, Alewood P, Jones A, Alewood D, Kent SBH. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Int J Pept Res Ther, 2007, 13: 31–44
Backes BJ, Virgilio AA, Ellman JA. Activation method to prepare a highly reactive acylsulfonamide “safety-catch” linker for solid-phase synthesis. J Am Chem Soc, 1996, 118: 3055–3056
Ingenito R, Bianchi E, Fattori D, Pessi A. Solid phase synthesis of peptide C-terminal thioesters by Fmoc/t-Bu chemistry. J Am Chem Soc, 1999, 121: 11369–11374
Macmillan D, Kang J. Peptide and protein thioester synthesis via N-to-S acyl transfer. Org Biomol Chem, 2010, 8: 1993–2002
Botti P, Manganiello S, Gaertner H. Native chemical ligation through in situ O to S acyl shift. Org Lett, 2004, 6: 4861–4864
Warren JD, Miller JS, Keding SJ, Danishefsky SJ. Toward fully synthetic glycoproteins by ultimately convergent routes: A solution to a long-standing problem. J Am Chem Soc, 2004, 126: 6576–6578
Zheng JS, Cui HK, Fang GM, Xi WX, Liu L. Chemical protein synthesis by kinetically controlled ligation of peptide O-esters. Chem-BioChem, 2010, 11: 511–515
Zheng JS, Xi WX, Wang FL, Guo QX, Li J. Fmoc-SPPS chemistry compatible approach for the generation of (glyco)peptide aryl thioesters. Tetrahedron Lett, 2011, 52: 2655–2660
Zheng JS, Chang HN, Wang FL, Liu L. Fmoc synthesis of peptide thioesters without post-chain-assembly manipulation. J Am Chem Soc, 2011, 133: 11080–11083
Fang GM, Li YM, Shen F, Huang YC, Li JB, Lin Y, Cui HK, Liu, L. Protein chemical synthesis by ligation of peptide hydrazides. Angew Chem Int Ed, 2011, 50: 7645–7649
Johnson ECB, Kent SBH. Insights into the mechanism and catalysis of the native chemical ligation reaction. J Am Chem Soc, 2006, 128: 6640–6646
Clark RJ, Craik D J. Native chemical ligation applied to the synthesis and bioengineering of circular peptides and proteins. Biopolymers, 2010, 94: 414–422
Craik DJ, Kaas Q. Analysis and classification of circular proteins in CyBase. Biopolymers, 2010, 94: 584–591
Shen F, Zhang ZP, Li JB, Lin Y, Liu L. Hydrazine-sensitive thiol protecting group for peptide and protein chemistry. Org Lett, 2011, 13: 568–571
Kimura RH, Tran AT, Camarero JA. Biosynthesis of the cyclotide Kalata B1 by using protein splicing. Angew Chem Int Ed, 2006, 45: 973–976
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, JS., Chang, HN., Shi, J. et al. Chemical synthesis of a cyclotide via intramolecular cyclization of peptide O-esters. Sci. China Chem. 55, 64–69 (2012). https://doi.org/10.1007/s11426-011-4434-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-011-4434-4