Abstract
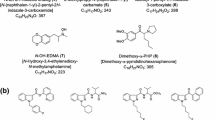
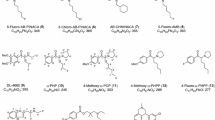
The changes in the prevalence of designer drugs and their legal status in Japan were investigated on the basis of the analyses of 686 different products containing synthetic cannabinoids and/or cathinone derivatives obtained from 2009 to February 2012. In the early stages of distribution of herbal-type products containing synthetic cannabinoids, cyclohexylphenols and naphthoylindoles were mostly found in the products. In November 2009, however, cannabicyclohexanol, CP-47,497 and JWH-018 were controlled as “designated substances” under the Pharmaceutical Affairs Law in Japan, and the cyclohexylphenols have since disappeared from the illegal drug market and been replaced by various analogs of the naphthoylindoles, phenylacetylindoles and benzoylindoles. These compounds, which have high affinities for the cannabinoid CB1 receptor, have become very popular, and the number of emergency hospitalizations associated with their use has dramatically increased from 2011. Other synthetic compounds with different structures and pharmacological effects, such as cathinone derivatives, have been detected together with the synthetic cannabinoids in herbal-type products since 2011. Moreover, many new types of synthetic cannabinoids, different from the four typical structures described, have also begun to appear since 2011. In addition to the synthetic cannabinoids, liquid or powdery-type products containing cathinone derivatives have been widely distributed recently. In 2009, the most popular cathinone derivative was 4-methylmethcathinone. After this compound was controlled as a designated substance in November 2009, cathinone derivatives, which have a pyrrolidine structure at the nitrogen atom and a 3,4-methylenedioxy structure, or analogs of 4-methylmethcathinone, became popular. In the present analysis, tryptamines were also detected in 31 % of the products containing cathinone derivatives. Local anesthetics such as procaine, lidocaine, benzocaine and dimethocaine were also frequently detected. In total, we identified at least 35 synthetic cannabinoids and 22 cathinone derivatives during this survey.




Similar content being viewed by others
References
Kikura-Hanajiri R, Uchiyama N, Kawamura M, Ogata J, Goda Y (2012) Prevalence of new designer drugs and their legal status in Japan (in Japanese with English abstract). Yakugaku Zasshi (in press)
Kikura-Hanajiri R, Uchiyama N, Goda Y (2011) Survey of current trends in the abuse of psychotropic substances and plants in Japan. Legal Med 13:109–115
Nagashima M, Seto T, Takahashi M, Miyake H, Yasuda I (2004) Screening method of the chemical illegal drugs by HPLC-PDA (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 55:67–71
Kikura-Hanajiri R, Hayashi M, Saisho K, Goda Y (2005) Simultaneous determination of 19 hallucinogenic tryptamines/β-calbolines and phenethylamines using GC–MS and LC-ESI–MS. J Chromatogr B 825:29–37
Seto T, Takahashi M, Nagashima M, Suzuki J, Yasuda I (2005) The identifications and the aspects of the commercially available uncontrolled drugs purchased between Apr. 2003 and Mar. 2004 (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 56:75–80
Matsumoto T, Kikura-Hanajiri R, Kamakura H, Kawamura N, Goda Y (2006) Identification of N-Methyl-4-(3,4-methylenedioxyphenyl)butan-2-amine, distributed as MBDB. J Health Sci 52:805–810
Takahashi M, Suzuki J, Nagashima M, Seto T, Yasuda I (2007) Newly detected compounds in uncontrolled drugs purchased in Tokyo between April 2006 and March 2007 (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 58:83–87
Kikura-Hanajiri R, Kawamura M, Saisho K, Kodama Y, Goda Y (2007) The disposition into hair of new designer drugs; methylone, MBDB and methcathinone. J Chromatogr B 855:121–126
Kikura-Hanajiri R, Kawamura M, Uchiyama N, Ogata J, Kamakura H, Saisho K, Goda Y (2008) Analytical data of designated substances (Shitei-Yakubutsu) controlled by the Pharmaceutical Affairs Law in Japan, part I: GC–MS and LC–MS (in Japanese with English abstract). Yakugaku Zasshi 128:971–979
Uchiyama N, Kawamura M, Kamakura H, Kikura-Hanajiri R, Goda Y (2008) Analytical data of designated substances (Shitei-Yakubutsu) controlled by the Pharmaceutical Affairs Law in Japan, part II: color test and TLC (in Japanese with English abstract). Yakugaku Zasshi 128:981–987
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2008) Analysis of designer drugs detected in the products purchased in fiscal year 2006 (in Japanese with English abstract). Yakugaku Zasshi 128:1499–1505
Suzuki J, Takahashi M, Seto T, Nagashima M, Okumoto C, Yasuda I (2006) Qualitative method of nitrite inhalants (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 57:115–120
Nagashima M, Seto T, Takahashi M, Suzuki J, Mori K, Ogino S (2008) Analyses of uncontrolled drugs purchased from April 2007 to March 2008 and newly found compounds (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 59:71–77
Uchiyama N, Miyazawa N, Kawamura M, Kikura-Hanajiri R, Goda Y (2010) Analysis of newly distributed designer drugs detected in the products purchased in fiscal year 2008 (in Japanese with English abstract). Yakugaku Zasshi 130:263–270
Nagashima M, Takahashi M, Suzuki J, Seto T, Mori K, Ogino S (2009) Analyses of uncontrolled drugs purchased Apr. 2008–Mar. 2009 (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 60:81–84
Kikura-Hanajiri R, Kawamura M, Miyajima A, Sunouchi M, Goda Y (2010) Determination of a new designer drug, N-hydroxy-3,4-methylenedioxymethamphetamine and its metabolites in rats using ultra-performance liquid chromatography-tandem mass spectrometry. Forensic Sci Int 198:62–69
Suzuki J, Moriyasu T, Nagashima M, Kanai C, Shimizu M, Hamano T, Nagayama T (2010) Analysis of uncontrolled drugs purchased in fiscal year 2009 (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 61:163–172
Uchiyama N, Kikura-Hanajiri R, Kawahara N, Goda Y (2009) Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol 27:61–66
Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y (2010) Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int 198:31–38
Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR (1993) Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265:218–226
Huffman JW (2009) Cannabimimetic indoles, pyrroles, and indenes: structure-activity relationships and receptor interactions. In: Reggio PH (ed) The cannabinoid receptors. Humana Press, New York, pp 49–94
Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y (2010) Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int 198:31–38
Nagashima M, Suzuki J, Moriyasu T, Yoshida M, Shimizu M, Hamano T, Nakae D (2011) Analysis of uncontrolled drugs purchased in the fiscal year 2010 (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 62:99–105
Yoshida M, Suzuki J, Takahashi M, Moriyasu T, Nakajima J, Kanai C, Nagashima M, Seto T, Shimizu M, Hamano T, Nakae D (2011) Investigations of designated substances detected from April 2010 to March 2011 (in Japanese). Ann Rep Tokyo Metrop Inst Public Health 62:107–114
Uchiyama N, Kikura-Hanajiri R, Shoda T, Fukuhara K, Goda Y (2011) Isomeric analysis of synthetic cannabinoids detected as designer drugs (in Japanese with English abstract). Yakugaku Zasshi 131:1141–1147
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2011) Identification and quantitative analyses of two cannabimimetic phenylacetylindoles, JWH-251 and JWH-250, and four cannabimimetic naphthoylindoles, JWH-081, JWH-015, JWH-200 and JWH-073, as designer drugs in illegal products. Forensic Toxicol 29:25–37
Uchiyama N, Kikura-Hanajiri R, Goda Y (2011) Identification of a novel cannabimimetic phenylacetylindole, cannabipiperidiethanone, as a designer drug in a herbal product and its affinity for cannabinoid CB1 and CB2 receptors. Chem Pharm Bull 59:1203–1205
Nakajima J, Takahashi M, Seto T, Suzuki J (2011) Identification and quantitation of cannabimimetic compound JWH-250 as an adulterant in products obtained via the Internet. Forensic Toxicol 29:51–55
Nakajima J, Takahashi M, Seto T, Kanai C, Suzuki J, Yoshida M, Hamano T (2011) Identification and quantitation of two benzoylindoles AM-694 and (4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone, and three cannabimimetic naphthoylindoles JWH-210, JWH-122, and JWH-019 as adulterants in illegal products obtained via the Internet. Forensic Toxicol 29:95–110
Nakajima J, Takahashi M, Nonaka R, Seto T, Suzuki J, Yoshida M, Kanai C, Hamano T (2011) Identification and quantitation of a benzoylindole (2-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone and a naphthoylindole 1-(5-fluoropentyl-1H-indol-3-yl)-(naphthalene-1-yl)methanone (AM-2201) found in illegal products obtained via the Internet and their cannabimimetic effects evaluated by in vitro [35S]GTPγS binding assays. Forensic Toxicol 29:132–141
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2012) Identification of two new-type synthetic cannabinoids, N-(1-adamantyl)-1-pentyl-1H-indole-3-carboxamide (APICA) and N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (APINACA), and detection of five synthetic cannabinoids, AM-1220, AM-2233, AM-1241, CB-13 (CRA-13) and AM-1248, as designer drugs in illegal products. Forensic Toxicol 30:114–125
Nakajima J, Takahashi M, Seto T, Yoshida M, Kanai C, Suzuki J, Hamano T (2012) Identification and quantitation of two new naphthoylindole drugs-of-abuse, (1-(5-hydroxypentyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone (AM-2202) and (1-(4-pentenyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone, with other synthetic cannabinoids in unregulated “herbal” products circulated in the Tokyo area. Forensic Toxicol 30:33–44
Uchiyama N, Matsuda S, Kawamura M, Kikura-Hanajiri R, Goda Y (2012) URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci Int, 2012 Oct 9. pii:S0379-0738(12)00434-3. doi:10.1016/j.forsciint.2012.08.047
Takahashi K, Uchiyama N, Fukiwake T, Hasegawa T, Saijou M, Motoki Y, Kikura-Hanajiri R, Goda Y (2012) Identification and quantitation of a cannabimimetic indole JWH-213 as a designer drug in an herbal product. Forensic Toxicol (in press)
Huffman JW, Mabon R, Wu MJ, Lu J, Hart R, Hurst DP, Reggio PH, Wiley JL, Martin BR (2003) 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB(1) cannabinoid receptor. Bioorg Med Chem 11:539–549
Makriyannis A, Deng H (2001) Cannabimimetic indole derivatives. WO patent 200128557
Makara JK, Mor M, Fegley D, Szabó SI, Kathuria S, Astarita G, Duranti A, Tontini A, Tarzia G, Rivara S, Freund TF, Piomelli D (2005) Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci 8:1139–1141
Acknowledgments
This research was supported by a Health Sciences Research Grant from the Ministry of Health, Labor and Welfare in Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is for the special issue TIAFT2012 edited by Osamu Suzuki.
Rights and permissions
About this article
Cite this article
Kikura-Hanajiri, R., Uchiyama, N., Kawamura, M. et al. Changes in the prevalence of synthetic cannabinoids and cathinone derivatives in Japan until early 2012. Forensic Toxicol 31, 44–53 (2013). https://doi.org/10.1007/s11419-012-0165-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11419-012-0165-2