Abstract
Purpose
Dissimilatory iron reduction is an important iron biogeochemical process in subsurface soils. Many researchers have studied the effects of various factors on this process. However, this process in the natural environment is complicated; thus, all the factors should be investigated systematically and simultaneously. The aims of this study were to investigate the effects of Fe(III) availability, surface areas, and crystallinity on Fe(III) reduction in different buffers and to characterize the surface properties of iron minerals.
Materials and methods
Microbial reductions of four chemically synthesized iron(III) oxyhydroxides [i.e., hydrous ferric oxide (HFO), α-FeOOH, γ-FeOOH, and α-Fe2O3] were conducted in batch cultures inoculated with an iron-reducing bacterium, Shewanella decolorationis S12, in Piperazine-1,4-bis(2-ethanesulfonic acid) (PIPES) and Na2HPO4/NaH2PO4 (phosphate buffer solution; PBS) buffers. The hydroxylamine hydrochloride extraction process was employed to determine Fe(III) availability of the oxyhydroxides, and their total surface area was measured using the Brunauer–Emmett–Teller method. Different α-Fe2O3 crystalline degrees were prepared by sintering γ-FeOOH powder from 300°C to 800°C. The surface properties of iron minerals before and after reduction were characterized using X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectra.
Results and discussion
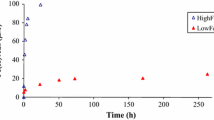
After 35 days of incubation, the order of Fe(III) reduction rates in the PIPES buffer was as follows: HFO > γ-FeOOH > α-FeOOH > α-Fe2O3, while that in the PBS buffer was as follows: HFO > α-FeOOH > γ-FeOOH > α-Fe2O3. The Michaelis–Menten rate expression was fit to describe the dependence of reduction rates on the available Fe(III). The reduction rates were positively correlated with the surface area but negatively correlated with the crystalline degree. The XRD and FTIR results coupled with the color changes in suspensions showed that in the PIPES buffer, HFO and γ-FeOOH were reduced to magnetite but no obvious change was found on the surfaces of α-FeOOH and α-Fe2O3. All iron oxyhydroxides were predominantly reduced to vivianite in the PBS buffer.
Conclusions
The present study suggested that S. decolorationis S12 can reduce different structural Fe(III) phases under anaerobic conditions. Iron minerals with higher Fe(III) availability and surface area can result in higher reduction rate, whereas high crystalline degree is not favorable for Fe(III) reduction. Magnetite was the dominant secondary mineral produced from the reduction of HFO and γ-FeOOH in the PIPES buffer, while that was vivianite upon the bioreduction of all iron oxyhydroxides in the PBS buffer.







Similar content being viewed by others
References
Bazylinski DA, Frankel RB, Konhauser KO (2007) Modes of biomineralization of magnetite by microbes. Geomicrobiol J 24:465–475
Behrends T, Van Cappellen P (2007) Transformation of hematite into magnetite during dissimilatory iron reduction-conditions and mechanisms. Geomicrobiol J 24:403–416
Bonneville S, Van Cappellen P, Behrends T (2004) Microbial reduction of iron(III) oxyhydroxides: effects of mineral solubility and availability. Chem Geol 212:255–268
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell A (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Borch T, Masue Y, Kukkadapu RK, Fendorf S (2007) Phosphate imposed limitations on biological reduction and alteration of ferrihydrite. Environ Sci Technol 41:166–172
Bridge TAM, Johnson DB (1998) Reduction of soluble iron and reductive dissolution of ferric iron-containing minerals by moderately thermophilic iron-oxidizing bacteria. Appl Environ Microbiol 64:2181–2186
Chi GY, Chen X, Shi Y, Zheng TH (2010) Forms and profile distribution of soil Fe in the Sanjiang Plain of Northeast China as affected by land uses. J Soil Sed 10:787–795
Feng CH, Li FB, Mai HJ, Li XZ (2010) Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment. Environ Sci Technol 44:1875–1880
Fredrickson JK, Gorby YA (1996) Environmental processes mediated by iron-reducing bacteria. Curr Opin Biotechnol 7:287–294
Fredrickson JK, Zachara JM, Kennedy DW, Dong HL, Onstott TC, Hinman NW, Li SM (1998) Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta 62:3239–3257
Glasauer S, Langley S, Beveridge TJ (2001) Sorption of Fe (hydr)oxides to the surface of Shewanella putrefaciens cell-bound fine-grained minerals are not always formed de novo. Appl Environ Microbiol 67:5544–5550
Glasauer S, Weidler PG, Langley S, Beveridge TJ (2003) Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim Cosmochim Acta 67:1277–1288
Hong YG, Xu MY, Guo J, Xu ZC, Chen XJ, Sun GP (2007) Respiration and growth of Shewanella decolorationis S12 with an azo compound as the sole electron acceptor. Appl Environ Microbiol 73:64–72
Jaisi DP, Dong H, Liu C (2007) Kinetic analysis of microbial reduction of Fe(III) in nontronite. Environ Sci Technol 41:2437–2444
Kostka JE, Wu J, Nealson KH, Stucki JW (1999) Effects of microbial reduction on physical and chemical properties of clay minerals. Geochim Cosmochim Acta 63:3705–3713
Kukkadapu RK, Zachara JM, Fredrickson JK, Kennedy DW (2004) Biotransformation of two-line silica-ferrihydrite by a dissimilatory Fe(III)-reducing bacterium: formation of carbonate green rust in the presence of phosphate. Geochim Cosmochim Acta 68:2799–2814
Legrand L, Mazerolles L, Chaussé A (2004) The oxidation of carbonate green rust into ferric phases: solid-state reaction or transformation via solution. Geochim Cosmochim Acta 683:497–3507
Li FB, Li XM, Zhou SG, Zhuang L, Cao F, Huang DY, Xu W, Liu TX, Feng CH (2010) Enhanced reductive dechlorination of DDT in an anaerobic system of dissimilatory iron-reducing bacteria and iron oxide. Environ Pollut 158:1733–1740
Li FB, Li XZ, Li XM, Liu TX, Dong J (2007) Heterogeneous photodegradation of bisphenol A with iron oxides and oxalate in aqueous solution. J Colloid Interface Sci 311:481–490
Li XM, Zhou SG, Li FB, Wu CY, Zhuang L, Xu W, Liu L (2009) Fe(III) oxide reduction and carbon tetrachloride dechlorination by a newly isolated Klebsiella pneumoniae strain L17. J Appl Microbiol 106:130–139
Liu C, Kota S, Zachara JM, Fredrickson JK, Brinkman CK (2001) Kinetic analysis of the bacterial reduction of goethite. Environ Sci Technol 35:2482–2490
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Lovley DR, Giovannoni SJ, White DC, Champine JE, Phillips EJP, Gorby YA, Goodwin S (1993) Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol 159:336–344
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
McCormick ML, Adriaens P (2004) Carbon tetrachloride transformation on the surface of nanoscale biogenic magnetite particles. Environ Sci Technol 38:1045–1053
Nevin KP, Holmes DE, Woodard TL, Hinlein ES, Ostendorf DW, Lovley DR (2005) Geobacter bemidjiensis sp. nov. and Geobacter psychrophilus sp. nov., two novel Fe(III)-reducing subsurface isolates. Int J Syst Evol Microbiol 55:1667–1674
Picardal FW, Arnold RG, Huey BB (1995) Effects of electron donor and acceptor conditions on reductive dehalogenation of tetrachloromethane by Shewanella putrefaciens 200. Appl Environ Microbiol 61:8–12
Roden EE (2003) Fe(III) oxide reactivity toward biological versus chemical reduction. Environ Sci Technol 37:1319–1324
Roden EE (2006) Geochemical and microbiological controls on dissimilatory iron reduction. CR Geosci 338:456–467
Roden EE, Urrutia MM (2002) Influence of biogenic Fe(II) on bacterial crystalline Fe(III) oxide reduction. Geomicrobiol J 19:209–251
Roden EE, Zachara JM (1996) Microbial reduction of crystalline iron(III) oxides influence of oxide surface area and potential for cell growth. Environ Sci Technol 30:1618–1628
Roh Y, Zhang CL, Vali H, Lauf RJ, Zhou J, Phelps TJ (2003) Biogeochemical and environmental factors in Fe biomineralization: magnetite and siderite formation. Clays Clay Miner 51:83–95
Scala DJ, Hacherl EL, Cowan R, Young LY, Kosson DS (2006) Characterization of Fe(III)-reducing enrichment cultures and isolation of Fe(III)-reducing bacteria. Res Microbiol 157:772–783
Schwertmann U, Cornell RM (1991) Iron oxides in the laboratory: preparation and characterization. VCH, New York
Slobodkin AI (2005) Thermophilic microbial metal reduction. Microbiology 74:501–514
Van Cappellen P, Gaillard JF, Rabouille C (1993) Biogeochemical transformations in sediments: kinetic models of early diagenesis. In: Wollast R, Mackenzie FT, Chou L (eds) Interactions of C, N, P and S biogeochemical cycles and global change. Springer, Berlin, pp 401–455
Wang XJ, Yang J, Chen XP, Sun GX, Zhu YG (2009) Phylogenetic diversity of dissimilatory ferric iron reducers in paddy soil of Hunan, South China. J Soil Sed 9:568–577
Xu MY, Guo J, Cen YH, Zhong XY, Cao W, Sun GP (2005) Shewanella decolorationis sp. nov., a dye-decolorizing bacterium isolated from activated sludge of a waste-water treatment plant. Int J Syst Evol Microbiol 55:363–368
Yan BZ, Wrenn BA, Basak S, Biswas P, Giammar DE (2008) Microbial reduction of Fe(III) in hematite nanoparticles by Geobacter sulfurreducens. Environ Sci Technol 42:6526–6531
Yang K, Peng HB, Wen YH, Li N (2010) Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl Surf Sci 256:3093–3097
Zachara JM, Fredrickson JK, Li SM, Kennedy DW, Smith SC, Gassman PL (1998) Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. Am Mineral 83:1426–1443
Zachara JM, Kukkadapu RK, Fredrickson JK, Gorby YA, Smith SC (2002) Biomineralization of poorly crystalline Fe(III) oxides by dissimilatory metal reducing bacteria (DMRB). Geomicrobiol J 19:179–207
Zegeye A, Ruby C, Jorand F (2007) Kinetic and thermodynamic analysis during dissimilatory γ-FeOOH reduction: formation of green rust 1 and magnetite. Geomicrobiol J 24:51–64
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 40901114, 41101217), the “973” Program (no. 2010CB134508)”, Guangzhou Technology Support Project (no. 2009Z1–E541), Natural Science Foundation of Guangdong Province (no. S2011040001094), and Excellent Young Scientist Foundation in Guangdong Academy of Sciences (2010).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Ji-Zheng He
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 741 kb)
Rights and permissions
About this article
Cite this article
Li, X., Liu, T., Li, F. et al. Reduction of structural Fe(III) in oxyhydroxides by Shewanella decolorationis S12 and characterization of the surface properties of iron minerals. J Soils Sediments 12, 217–227 (2012). https://doi.org/10.1007/s11368-011-0433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-011-0433-5