Abstract
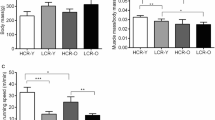
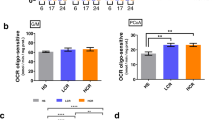
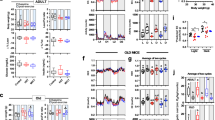
Age-associated declines in aerobic capacity promote the development of various metabolic diseases. In rats selectively bred for high/low intrinsic aerobic capacity, greater aerobic capacity reduces susceptibility to metabolic disease while increasing longevity. However, little remains known how intrinsic aerobic capacity protects against metabolic disease, particularly with aging. Here, we tested the effects of aging and intrinsic aerobic capacity on systemic energy expenditure, metabolic flexibility and mitochondrial protein synthesis rates using 24-month-old low-capacity (LCR) or high-capacity runner (HCR) rats. Rats were fed low-fat diet (LFD) or high-fat diet (HFD) for eight weeks, with energy expenditure (EE) and metabolic flexibility assessed utilizing indirect calorimetry during a 48 h fast/re-feeding metabolic challenge. Deuterium oxide (D2O) labeling was used to assess mitochondrial protein fraction synthesis rates (FSR) over a 7-day period. HCR rats possessed greater EE during the metabolic challenge. Interestingly, HFD induced changes in respiratory exchange ratio (RER) in male and female rats, while HCR female rat RER was largely unaffected by diet. In addition, analysis of protein FSR in skeletal muscle, brain, and liver mitochondria showed tissue-specific adaptations between HCR and LCR rats. While brain and liver protein FSR were altered by aerobic capacity and diet, these effects were less apparent in skeletal muscle. Overall, we provide evidence that greater aerobic capacity promotes elevated EE in an aged state, while also regulating metabolic flexibility in a sex-dependent manner. Modulation of mitochondrial protein FSR by aerobic capacity is tissue-specific with aging, likely due to differential energetic requirements by each tissue.






Similar content being viewed by others
References
Myers J, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801.
Hawkins MN, et al. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med Sci Sports Exerc. 2007;39(1):103–7.
Rogers MA, et al. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68(5):2195–9.
Betik AC, Hepple RT. Determinants of VO2 max decline with aging: an integrated perspective. Appl Physiol Nutr Metab. 2008;33(1):130–40.
Fleg JL, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–82.
Pontzer H, et al. Daily energy expenditure through the human life course. Science. 2021;373(6556):808–12.
Manini TM. Energy expenditure and aging. Ageing Res Rev. 2010;9(1):1–11.
Hill JO, Wyatt HR, Peters JC. The importance of energy balance. Eur Endocrinol. 2013;9(2):111–5.
Yasukata J, et al. Relationship between measured aerobic capacity and total energy expenditure obtained by the doubly labeled water method in community-dwelling, healthy adults aged 81–94 years. Geriatrics (Basel). 2022;7(2):48.
Roberts SB, Dallal GE. Effects of age on energy balance. Am J Clin Nutr. 1998;68(4):975S-979S.
Amdanee N, et al. Age-associated changes of resting energy expenditure, body composition and fat distribution in Chinese Han males. Physiol Rep. 2018;6(23):e13940.
Bosy-Westphal A, et al. The Age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J Nutr. 2003;133(7):2356–62.
Goodpaster BH, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64.
Paez HG, Pitzer CR, Alway SE. Age-related dysfunction in proteostasis and cellular quality control in the development of sarcopenia. Cells. 2023;12(2):249.
Short KR, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618-23.
Markaki M, Tavernarakis N. Mitochondrial turnover and homeostasis in ageing and neurodegeneration. FEBS Lett. 2020;594(15):2370–9.
Rooyackers OE, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364-9
Menshikova EV, et al. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–40.
Wisloff U, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–20.
Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5(1):45–52.
Thyfault JP, Morris EM. Intrinsic (genetic) aerobic fitness impacts susceptibility for metabolic disease. Exerc Sport Sci Rev. 2017;45(1):7–15.
Morris EM, et al. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J Physiol. 2017;595(14):4909–26.
Morris EM, et al. Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2016;311(4):E749-e760.
Britton SL, Koch LG. Animal genetic models for complex traits of physical capacity. Exerc Sport Sci Rev. 2001;29(1):7–14.
Thyfault JP, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–16.
Morris EM, et al. Difference in housing temperature-induced energy expenditure elicits sex-specific diet-induced metabolic adaptations in mice. Obesity (Silver Spring). 2020;28(10):1922–31.
Miller BF, et al. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol. 2018;596(1):83–103.
Morris EM, et al. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol-Endocrinol Metab. 2014;307(4):E355–64.
Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr. 1986;44(2):173–80.
Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1097–105.
Seifert EL, et al. Intrinsic aerobic capacity correlates with greater inherent mitochondrial oxidative and H2O2 emission capacities without major shifts in myosin heavy chain isoform. J Appl Physiol (1985). 2012;113(10):1624–34.
Bouchard C, Blair SN, Katzmarzyk PT. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc. 2015;90(11):1533–40.
Koch LG, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109(10):1162–72.
Church TS, et al. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–20.
Mandsager K, et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6):e183605–e183605.
Reimers CD, Knapp G, Reimers AK. Does physical activity increase life expectancy? A review of the literature. J Aging Res. 2012;2012:243958.
Morris EM, et al. Intrinsic high aerobic capacity in male rats protects against diet-induced insulin resistance. Endocrinology. 2019;160(5):1179–92.
Naples SP, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab. 2010;35(2):151–62.
Goodpaster BH, et al. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–61.
Haus JM, et al. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98(7):E1181–8.
Aon MA, et al. Mitochondrial health is enhanced in rats with higher vs. lower intrinsic exercise capacity and extended lifespan. NPJ Aging Mech Dis. 2021;7(1):1.
Henry CJK. Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr. 2000;54(3):S77–91.
Monferrer-Marín J, et al. Impact of ageing on female metabolic flexibility: A cross-sectional pilot study in over-60 active women. Sports Med - Open. 2022;8(1):97.
Morris EM, et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1α overexpression. Am J Physiol Gastrointest Liver Physiol. 2013;305(11):G868–80.
Jackson AS, et al. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169(19):1781–7.
Bouchard C, Rankinen T, Timmons JA. Genomics and genetics in the biology of adaptation to exercise. Compr Physiol. 2011;1(3):1603–48.
Mukherjee SD, et al. Aerobic capacity modulates adaptive thermogenesis: Contribution of non-resting energy expenditure. Physiol Behav. 2020;225:113048.
Gavini CK, et al. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306(6):E635–47.
Son C, et al. Reduction of diet-induced obesity in transgenic mice overexpressing uncoupling protein 3 in skeletal muscle. Diabetologia. 2004;47(1):47–54.
Fink BD, et al. Mitochondrial proton leak in obesity-resistant and obesity-prone mice. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1773–80.
Matthew Morris E, et al. Increased aerobic capacity reduces susceptibility to acute high-fat diet-induced weight gain. Obesity (Silver Spring). 2016;24(9):1929–37.
Smith RL, et al. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev. 2018;39(4):489–517.
Coen PM, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol: Ser A. 2012;68(4):447–55.
Lagerwaard B, et al. In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur J Appl Physiol. 2019;119(8):1799–808.
Moehle EA, Shen K, Dillin A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J Biol Chem. 2019;294(14):5396–407.
Grevendonk L, et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat Commun. 2021;12(1):4773.
Lima T, et al. Pleiotropic effects of mitochondria in aging. Nature Aging. 2022;2(3):199–213.
Keele GR, Zhang JG, Szpyt J, Korstanje R, Gygi SP, Churchill GA, Schweppe DK. Global and tissue-specific aging effects on murine proteomes. Cell Rep. 2023;42:112715.
Robinson MM, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25(3):581–92.
Baar K, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Faseb j. 2002;16(14):1879–86.
Vieira-Potter VJ, et al. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;308(6):R530–42.
Stephenson EJ, et al. Exercise training enhances white adipose tissue metabolism in rats selectively bred for low- or high-endurance running capacity. Am J Physiol Endocrinol Metab. 2013;305(3):E429–38.
Choi J, et al. Brain diabetic neurodegeneration segregates with low intrinsic aerobic capacity. Ann Clin Transl Neurol. 2014;1(8):589–604.
Gan L, et al. Region-specific differences in bioenergetic proteins and protein response to acute high fat diet in brains of low and high capacity runner rats. Neurosci Lett. 2018;674:49–53.
Kaliszewska A, et al. The interaction of diet and mitochondrial dysfunction in aging and cognition. Int J Mol Sci. 2021;22(7):3574.
Wikgren J, et al. Selective breeding for endurance running capacity affects cognitive but not motor learning in rats. Physiol Behav. 2012;106(2):95–100.
Acknowledgements
Edziu Franczak was involved in the investigation, statistical analysis, visualization, and writing of the original draft. Adrianna Maurer was involved in the investigation, methodology, and writing-review and editing. Vivien Csikos Drummond, Emily Wells, Madi Wenger, Frederick F. Peelor III, Abby Crosswhite, and Benjamin F. Miller were involved in the investigation, statistical analysis, visualization, and writing-review and editing, Colin S. McCoin and Benjamin A. Kugler were involved in the investigation and writing-review and editing. Steven L. Britton and Lauren G. Koch were involved in the development of rat models and writing-review. John P. Thyfault was involved in the conceptualization, methodology, formal analysis, investigation, funding acquisition, and writing-review and editing.
Funding
This project was supported in part by National Institutes of Health R01DK121497 (JPT) and R01DK121497-03A1 Supplement (JPT), and the National Institute of General Medical Sciences S10OD028598 (JPT). The HCR/LCR rat models were funded by National Institutes of Health Office of Research Infrastructure Programs Grant P40OD-021331 and 3P40OD021331-06S1 (LGK and SLB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11357_2023_985_MOESM1_ESM.tiff
Supplemental Fig. 1. Representative images of hepatic triglyceride storage. Representative images of Hematoxylin and eosin staining on liver sections in male and female HCR rats fed either a HFD or LFD (TIFF 163854 KB)
11357_2023_985_MOESM2_ESM.tiff
Supplemental Fig. 2. Skeletal muscle and liver correlations between energy expenditure and mitochondrial protein fractional synthesis rates. Correlations between total EE and mitochondrial protein FSR in (a) male and (b) female gastrocnemius muscle, soleus (c, d) and liver (e, f) (TIFF 4004 KB)
About this article
Cite this article
Franczak, E., Maurer, A., Drummond, V.C. et al. Divergence in aerobic capacity and energy expenditure influence metabolic tissue mitochondrial protein synthesis rates in aged rats. GeroScience 46, 2207–2222 (2024). https://doi.org/10.1007/s11357-023-00985-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00985-1