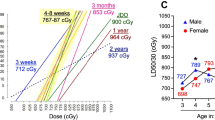
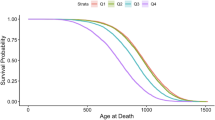
Abstract
The Wake Forest nonhuman primate (NHP) Radiation Late Effects Cohort (RLEC) is a unique and irreplaceable population of aging NHP radiation survivors which serves the nation’s need to understand the late effects of radiation exposure. Over the past 16 years, Wake Forest has evaluated > 250 previously irradiated rhesus macaques (Macaca mulatta) that were exposed to single total body irradiation (IR) doses of 1.14–8.5 Gy or to partial body exposures of up to 10 Gy (5% bone marrow sparing) or 10.75 Gy (whole thorax). Though primarily used to examine IR effects on disease-specific processes or to develop radiation countermeasures, this resource provides insights on resilience across physiologic systems and its relationship with biological aging. Exposure to IR has well documented deleterious effects on health, but the late effects of IR are highly variable. Some animals exhibit multimorbidity and accumulated health deficits, whereas others remain relatively resilient years after exposure to total body IR. This provides an opportunity to evaluate biological aging at the nexus of resilient/vulnerable responses to a stressor. Consideration of inter-individual differences in response to this stressor can inform individualized strategies to manage late effects of radiation exposure, and provide insight into mechanisms underlying systemic resilience and aging. The utility of this cohort for age-related research questions was summarized at the 2022 Trans-NIH Geroscience Interest Group’s Workshop on Animal Models for Geroscience. We present a brief review of radiation injury and its relationship to aging and resilience in NHPs with a focus on the RLEC.


Similar content being viewed by others
References
Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12(1):8–21. https://doi.org/10.1016/j.arr.2012.03.010.
Justice JN, Cesari M, Seals DR, Shively CA, Carter CS. Comparative approaches to understanding the relation between aging and physical function. J Gerontol a-Biol. 2016;71(10):1243–53. https://doi.org/10.1093/gerona/glv035.
Rogers J, Gibbs RA. Comparative primate genomics: emerging patterns of genome content and dynamics. Nat Rev Genet. 2014;15(5):347–59. https://doi.org/10.1038/nrg3707.
Walker ML, Gordon TP, Wilson ME. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod. 1983;29(4):841–8. https://doi.org/10.1095/biolreprod29.4.841.
Simmons HA. Age-associated pathology in rhesus macaques (Macaca mulatta). Vet Pathol. 2016;53(2):399–416. https://doi.org/10.1177/0300985815620628.
Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. https://doi.org/10.1146/annurev-animal-022114-110829.
Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mamm Genome. 2016;27(7–8):279–88. https://doi.org/10.1007/s00335-016-9638-7.
Freire-Cobo C, Rothwell ES, Varghese M, Edwards M, Janssen WGM, Lacreuse A, et al. Neuronal vulnerability to brain aging and neurodegeneration in cognitively impaired marmoset monkeys (Callithrix jacchus). Neurobiol Aging. 2023;123:49–62. https://doi.org/10.1016/j.neurobiolaging.2022.12.001.
Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52(1):54–65. https://doi.org/10.1093/ilar.52.1.54.
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
Fraser HC, Kuan V, Johnen R, Zwierzyna M, Hingorani AD, Beyer A, et al. Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell. 2022;21(4):e13524. https://doi.org/10.1111/acel.13524.
Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71(11):1407–14. https://doi.org/10.1093/gerona/glw086.
Whitson HE, Cohen HJ, Schmader KE, Morey MC, Kuchel G, Colon-Emeric CS. Physical resilience: not simply the opposite of frailty. J Am Geriatr Soc. 2018;66(8):1459–61. https://doi.org/10.1111/jgs.15233.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. https://doi.org/10.1093/gerona/56.3.m146.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–95. https://doi.org/10.1503/cmaj.050051.
Hadley EC, Kuchel GA, Newman AB, Workshop S, Participants. Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72(7):980–90. https://doi.org/10.1093/gerona/glx015.
LeBrasseur NK. Physical resilience: opportunities and challenges in translation. J Gerontol A Biol Sci Med Sci. 2017;72(7):978–9. https://doi.org/10.1093/gerona/glx028.
Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71(4):489–95. https://doi.org/10.1093/gerona/glv202.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. https://doi.org/10.1016/j.cell.2013.05.039.
Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Evaluating health span in preclinical models of aging and disease: guidelines, challenges, and opportunities for geroscience. J Gerontol A Biol Sci Med Sci. 2016;71(11):1395–406. https://doi.org/10.1093/gerona/glw106.
Sedrak MS, Gilmore NJ, Carroll JE, Muss HB, Cohen HJ, Dale W. Measuring biologic resilience in older cancer survivors. J Clin Oncol. 2021;39(19):2079–89. https://doi.org/10.1200/JCO.21.00245.
Calabrese EJ. Hormesis mediates acquired resilience: using plant-derived chemicals to enhance health. Annu Rev Food Sci Technol. 2021;12:355–81. https://doi.org/10.1146/annurev-food-062420-124437.
Epel ES. The geroscience agenda: toxic stress, hormetic stress, and the rate of aging. Ageing Res Rev. 2020;63:101167. https://doi.org/10.1016/j.arr.2020.101167.
Calabrese EJ, Baldwin LA. Radiation hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19(1):41–75. https://doi.org/10.1191/096032700678815602.
Al-Jumayli M, Brown SL, Chetty IJ, Extermann M, Movsas B. The biological process of aging and the impact of ionizing radiation. Semin Radiat Oncol. 2022;32(2):172–8. https://doi.org/10.1016/j.semradonc.2021.11.011.
Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140(12):1037–51. https://doi.org/10.7326/0003-4819-140-12-200406150-00015.
Dainiak N, Albanese J. Medical management of acute radiation syndrome. J Radiol Prot. 2022;42(3). https://doi.org/10.1088/1361-6498/ac7d18.
Zablotska LB, Bazyka D, Lubin JH, Gudzenko N, Little MP, Hatch M, et al. Radiation and the risk of chronic lymphocytic and other leukemias among chornobyl cleanup workers. Environ Health Perspect. 2013;121(1):59–65. https://doi.org/10.1289/ehp.1204996.
Ron E, Preston DL, Mabuchi K, Thompson DE, Soda M. Cancer incidence in atomic bomb survivors. Part IV: comparison of cancer incidence and mortality. Radiat Res. 1994;137(2 Suppl):S98-112.
Douple EB, Mabuchi K, Cullings HM, Preston DL, Kodama K, Shimizu Y, et al. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep. 2011;5(Suppl 1 (0 1)):S122-33. https://doi.org/10.1001/dmp.2011.21.
Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: a 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64(4):1081–91. https://doi.org/10.1016/j.ijrobp.2005.10.022.
Wakeford R. The cancer epidemiology of radiation. Oncogene. 2004;23(38):6404–28. https://doi.org/10.1038/sj.onc.1207896.
Upton AC, Kimball AW, Furth J, Christenberry KW, Benedict WH. Some delayed effects of atom-bomb radiations in mice. Cancer Res. 1960;20((8)Pt2):1–60.
Strehler BL. Origin and comparison of the effects of time and high-energy radiations on living systems. Q Rev Biol. 1959;34(2):117–42. https://doi.org/10.1086/402632.
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. https://doi.org/10.1111/acel.12344.
Miousse IR, Kutanzi KR, Koturbash I. Effects of ionizing radiation on DNA methylation: from experimental biology to clinical applications. Int J Radiat Biol. 2017;93(5):457–69. https://doi.org/10.1080/09553002.2017.1287454.
Fanning KM, Pfisterer B, Davis AT, Presley TD, Williams IM, Wasserman DH, et al. Changes in microvascular density differentiate metabolic health outcomes in monkeys with prior radiation exposure and subsequent skeletal muscle ECM remodeling. Am J Physiol Regul Integr Comp Physiol. 2017;313(3):R290–7. https://doi.org/10.1152/ajpregu.00108.2017.
DeBo RJ, Lees CJ, Dugan GO, Caudell DL, Michalson KT, Hanbury DB, et al. Late effects of total-body gamma irradiation on cardiac structure and function in male rhesus macaques. Radiat Res. 2016;186(1):55–64. https://doi.org/10.1667/RR14357.1.
Michalson KT, Macintyre AN, Sempowski GD, Bourland JD, Howard TD, Hawkins GA, et al. Monocyte polarization is altered by total-body irradiation in male rhesus macaques: implications for delayed effects of acute radiation exposure. Radiat Res. 2019;192(2):121–34. https://doi.org/10.1667/RR15310.1.
French MJ, Wuerker R, Dugan G, Olson JD, Sanders BR, Tooze JA, et al. Long-term immunological consequences of radiation exposure in a diverse cohort of rhesus macaques. Int J Radiat Oncol Biol Phys. 2022. https://doi.org/10.1016/j.ijrobp.2022.10.024.
Macintyre AN, French MJ, Sanders BR, Riebe KJ, Shterev ID, Wiehe K, et al. Long-term recovery of the adaptive immune system in rhesus macaques after total body irradiation. Adv Radiat Oncol. 2021;6(5):100677. https://doi.org/10.1016/j.adro.2021.100677.
Ruggiero AD, Davis MA, Davis AT, DeStephanis D, Williams AG, Vemuri R, et al. Delayed effects of radiation in adipose tissue reflect progenitor damage and not cellular senescence. Geroscience. 2022. https://doi.org/10.1007/s11357-022-00660-x.
Kavanagh K, Dendinger MD, Davis AT, Register TC, DeBo R, Dugan G, et al. Type 2 diabetes is a delayed late effect of whole-body irradiation in nonhuman primates. Radiat Res. 2015;183(4):398–406. https://doi.org/10.1667/Rr13916.1.
Bacarella N, Ruggiero A, Davis AT, Uberseder B, Davis MA, Bracy DP, et al. Whole body irradiation induces diabetes and adipose insulin resistance in nonhuman primates. Int J Radiat Oncol Biol Phys. 2020;106(4):878–86. https://doi.org/10.1016/j.ijrobp.2019.11.034.
Hale LP, Rajam G, Carlone GM, Jiang C, Owzar K, Dugan G, et al. Late effects of total body irradiation on hematopoietic recovery and immune function in rhesus macaques. PLoS One. 2019;14(2):e0210663. https://doi.org/10.1371/journal.pone.0210663.
Andrews RN, Bloomer EG, Olson JD, Hanbury DB, Dugan GO, Whitlow CT, et al. Non-human primates receiving high-dose total-body irradiation are at risk of developing cerebrovascular injury years postirradiation. Radiat Res. 2020;194(3):277–87. https://doi.org/10.1667/RADE-20-00051.1.
Andrews RN, Caudell DL, Metheny-Barlow LJ, Peiffer AM, Tooze JA, Bourland JD, et al. Fibronectin produced by cerebral endothelial and vascular smooth muscle cells contributes to perivascular extracellular matrix in late-delayed radiation-induced brain injury. Radiat Res. 2018;190(4):361–73. https://doi.org/10.1667/Rr14961.1.
Andrews RN, Dugan GO, Peiffer AM, Hawkins GA, Hanbury DB, Bourland JD, et al. White matter is the predilection site of late-delayed radiation-induced brain injury in non-human primates. Radiat Res. 2019;191(3):217–31. https://doi.org/10.1667/RR15263.1.
Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, et al. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 2017;187(5):599–611. https://doi.org/10.1667/Rr14616.1.
Hanbury DB, Peiffer AM, Dugan G, Andrews RN, Clinc JM. Long-term cognitive functioning in single-dose total-body gamma-irradiated rhesus monkeys (Macaca mulatta). Radiat Res. 2016;186(5):447–54. https://doi.org/10.1667/Rr14430.1.
Farris M, McTyre ER, Okoukoni C, Dugan G, Johnson BJ, Blackstock AW, et al. Cortical thinning and structural bone changes in non-human primates after single-fraction whole-chest irradiation. Radiat Res. 2018;190(1):63–71. https://doi.org/10.1667/RR15007.1.
Little MP, Brenner AV, Grant EJ, Sugiyama H, Preston DL, Sakata R, et al. Age effects on radiation response: summary of a recent symposium and future perspectives. Int J Radiat Biol. 2022;1–11. https://doi.org/10.1080/09553002.2022.2063962.
Sills WS, Tooze JA, Olson JD, Caudell DL, Dugan GO, Johnson BJ, et al. Total-body irradiation is associated with increased incidence of mesenchymal neoplasia in a radiation late effects cohort of rhesus macaques (Macaca mulatta). Int J Radiat Oncol Biol Phys. 2022;113(3):661–74. https://doi.org/10.1016/j.ijrobp.2022.02.019.
Cohen EP, Olson JD, Tooze JA, Bourland JD, Dugan GO, Cline JM. Detection and quantification of renal fibrosis by computerized tomography. PLoS One. 2020;15(2):e0228626. https://doi.org/10.1371/journal.pone.0228626.
Acknowledgements
We thank the laboratory and veterinary technicians and research assistants who conducted assessments. We also give special thanks to John Olson at WFSM for assistance with the animal cohort, and data retrieval, and members of the Register Laboratory for performing clinical biochemical assays.
Funding
This work was supported by the National Institutes of Health (NIH) program grant to Wake Forest University School of Medicine to support the NIAID-funded Radiation Survivor Cohort, U01 AI150578, (Drs. Cline and Schaaf), and the NIA-supported administrative supplement to evaluate aging outcomes and biological mechanism in this cohort. The authors were also supported by career development grants K01 AG059837 (Dr. Justice), and K01 AG056663 (Dr. Quillen).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All Radiation Late Effects Cohort experimental animal work is conducted at the Wake Forest University School of Medicine, an AAALAC-accredited institution, with IACUC approval.
Consent for publication
All authors consent to publication in GeroScience.
Conflict of interest
Drs. Cline and Schaaf have funding from Roche Pharmaceutical for work unrelated to this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Schaaf, G.W., Justice, J.N., Quillen, E.E. et al. Resilience, aging, and response to radiation exposure (RARRE) in nonhuman primates: a resource review. GeroScience 45, 3371–3379 (2023). https://doi.org/10.1007/s11357-023-00812-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-023-00812-7