Abstract
The molecular mechanisms underlying cognitive decline during healthy aging remain largely unknown. Utilizing aged wild-type C57BL/6 mice as a model for normal aging, we tested the hypothesis that cognitive performance, memory, and learning as assessed in established behavioral testing paradigms are correlated with the differential expression of isoforms of the Homer family of synaptic scaffolding proteins. Here we describe a loss of cognitive and motor function that occurs when Homer-1a/Vesl-1S protein levels drop during aging. Our data describe a novel mechanism of age-related synaptic changes contributing to loss of biological function, spatial learning, and memory formation as well as motor coordination, with the dominant negative uncoupler of synaptic protein clustering, Homer-1a/Vesl-1S, as a potential target for the prophylaxis and treatment of age-related cognitive decline.
Similar content being viewed by others
Introduction
Slow, progressive impairments of cognitive and motor function are typically associated with aging, even in the absence of diagnosable neurodegenerative conditions, such as dementia, Alzheimer’s, or Parkinson’s disease (Erickson and Barnes 2003; Mufson et al. 2012). Progressive accumulation of changes in the synaptic structure and function at the molecular level, ultimately leading to the loss of biological function, is generally considered the underlying etiology (Hayflick 2007); however, the exact molecular and cellular mechanisms are only poorly understood.
Scaffolding proteins play a critical role in maintaining synaptic integrity by organizing synaptic proteins (Aarts et al. 2003; Hayashi et al. 2009; Haucke et al. 2011). Vesl/Homer proteins are a group of scaffolding proteins that facilitate the effective conversion of extracellular into intracellular signals at excitatory synapses by clustering and organizing signaling proteins at synaptic sites. The Homer-1 gene is alternatively spliced into four known isoforms. The short isoform Vesl-1S/Homer-1a is an immediate early gene product that is rapidly and transiently induced by high synaptic activity, including during seizures and long-term potentiation (Brakeman et al. 1997; Kato et al. 1998; Duncan et al. 2005), while the long Homer-1 isoforms are typically expressed constitutively (Duncan et al. 2005).
Homer proteins possess an Ena/vasodilator-stimulated phosphoprotein homology-1 domain through which Homer binds to its interaction partners (Hayashi et al. 2009). The long Homer isoforms also have a C-terminal coiled-coil (CC) domain, which mediates the multimerization among Homer monomers resulting in the regulation of protein function (Hayashi et al. 2009). The CC domain is absent in the Homer-1a isoform suggesting that this isoform, which is therefore incapable of multimerization, has the potential to act as a dominant negative regulator. In fact, Homer-1a competes with the long isoforms for binding sites on Homer-1 ligands, thereby providing a stimulus-dependent mechanism using the molecular determinants of Vesl/Homer proteins to regulate intracellular signaling pathways (Duncan et al. 2005).
Changes in Vesl-1L/Homer-1c have been associated with aging and injury. Specifically, we have previously described loss of Vesl-1L/Homer-1c as biomarker for synaptic changes after injury in the retina (Kaja et al. 2003), and experimental down-regulation of Homer-1b/c has been shown to attenuate glutamate-mediated toxicity in cortical neurons in vitro (Chen et al. 2012).
It is well-established that the neurobiology of memory and learning changes during aging (Erickson and Barnes 2003). The changes are a progressive, albeit slow decline in cognitive and motor function and also occur in the absence of diagnosable neurodegenerative conditions (Mufson et al. 2012). In studies of human aging, correlating cognitive function with postmortem brain pathology is one of the few approaches available; however, genetic differences between individuals, lack of standardized testing and the availability of postmortem tissue greatly diminish the feasibility of this approach. In contrast, rodent models offer a battery of reliable behavioral tests for cognitive and psychomotor function (Collier and Coleman 1991; Nguyen 2006). This approach is based on the existence of individual differences in the degree of functional impairment among relatively old mice that are chronologically of the same age (Dubey et al. 1996; Forster et al. 1996; Sumien et al. 2004; McDonald and Forster 2005), despite a shared genetic background (Nguyen 2006; Brayton et al. 2012). This is generally thought to result from the accumulation of changes in synaptic structure and function at the molecular level, ultimately leading to the loss of biological function (Hayflick 2007). As such, individual differences reflect the differential progression of underlying age-related brain changes in individuals and have been used extensively as a tool for identifying the specific anatomical, cellular, and molecular substrates involved in age-related cognitive decline (Rapp and Amaral 1992; Baxter and Gallagher 1996; Ingram 1996; Gallagher and Rapp 1997; Calhoun et al. 1998; Nicholson et al. 2004).
In the present study, we chose 6- and 24-month-old C57BL/6 mice, a widely used model of normal aging (Brayton et al. 2012), and describe a highly significantly correlation of forebrain Homer-1a expression with deficits in spatial learning and memory formation as well as in motor coordination. Our data describe a novel mechanism of age-related synaptic changes contributing to loss of biological function and identify Homer-1a as a potential target for the prophylaxis and treatment of age-related cognitive decline.
Methods
Animals
All animal experiments were approved by the local Institutional Animal Care and Use Committee. Ten young (6 months) and ten old (24 months) male C57BL/6J mice were obtained from the National Institute on Aging and subsequently maintained individually in the institutional vivarium at ambient temperature (23 ± 1 °C), under a 12-h light/dark cycle starting at 0600 hours. Mice had ad libitum access to food and water except during the testing sessions.
Behavioral assays
Spatial learning and memory. Spatial learning and memory were measured using a swim maze test as described previously (Sumien et al. 2006). Mice were allowed to swim in a steel tank (110 cm diameter, 60 cm deep), filled with opaque water, maintained at 24 ± 1 °C. A hidden submersed platform (1.5 cm below the surface) was provided as an escape. A computerized tracking system (Anymaze, Stoelting Inc., Chicago, IL, USA) recorded the path taken and the swimming speed of the animal. During the pretraining phase, mice learned the motor components of swimming and climbing onto the platform, without learning its location in the tank. Subsequently, mice were tested for their ability to learn the location of the platform during four acquisition sessions. Each session consisted of five trials during which the mouse had to swim to the platform from a different starting point in the tank. Performance in the swim maze test was used to determine the Learning Index, which we defined as the relative improvement between the first and the last session, normalized to average performance of young animals using path length as quantitative measure.
Bridge walking. Each mouse was tested for the latency (seconds) to fall after being placed on one of four bridges (2 cm square, 2 cm round, 1 cm square, 1 cm round), mounted 45 cm above a padded surface. The four bridges differed in their diameter (small vs. large) and shape (round or square), thus providing four levels of difficulty. The maximal latency to fall was set at 60 s. Each bridge was presented three times and the mean latency to fall was used as measure of performance for each bridge.
Antibodies
The following previously validated primary antibodies were used: goat anti-Homer-1a (1:100; sc-8922) (Giuffrida et al. 2005), rabbit anti-Homer-1b/c (1:100; sc-20807) (Ghasemzadeh et al. 2009), and rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000; sc-25778), all from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Secondary antibodies were horseradish peroxidase (HRP)-linked donkey-anti rabbit IgG-HRP (1:10,000; NA9340, GE Healthcare, Piscataway, NJ, USA) and donkey anti-goat IgG-HRP (1:5,000; sc-2020, Santa Cruz Biotechnology).
Quantitative immunoblotting
Animals were euthanized using carbon dioxide asphyxiation. Brains were dissected into forebrain, olfactory bulb, and cerebellum and snap frozen in liquid nitrogen. The protein concentration was determined according to the method of Lowry et al. (1951). Twenty-five micrograms of total protein were separated electrophoretically and transferred to nitrocellulose membranes (0.22 μm; Pall Corp., Port Washington, NY, USA), as described previously (Kaja et al. 2011). Membranes were imaged on a G-Box imaging station (Syngene, Oxford, UK). Quantification was performed by densitometry using ImageJ software (NIH, Bethesda, MD, USA). Density values were corrected for background and Homer-1 expression normalized for endogenous GAPDH expression.
Analysis and statistics
Data were analyzed in Prism 5.01 software (GraphPad Software Inc., La Jolla, CA, USA). Groups were compared using Student’s t test. Correlation analysis was performed as described by us previously (Burroughs et al. 2011), calculating a Pearson product-moment correlation coefficient (r) to evaluate the strength of the association. Outlier analysis was performed on values of normalized Homer-1 expression using a difference of two standard deviations from the mean as the exclusion criterion. In total, one value out of 20 was excluded from the analysis.
Results
Decline in spatial learning and memory formation as well as motor coordination with age
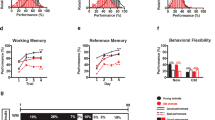
In order to quantify cognitive function, we used established surrogate testing paradigms and measures for learning. In the swim maze test, 6-month-old (young) mice showed statistically significant improved performance as assessed by path length to successfully reaching the hidden platform starting from the third session (Fig. 1a). By the fourth session, path length had decreased by 62 % from 844 ± 95 to 323 ± 77 cm (P < 0.01; Fig. 1a). In contrast, 24-month-old (aged) mice showed a similar performance as young mice during the first session (968 ± 87 cm); however, they did not show a statistically significant improvement; the performance of aged mice (697 ± 87 cm) during the fourth session was significantly poorer than that of the young cohort (P < 0.01; Fig. 1a). Similar differences were observed when comparing the two cohorts based on latency to reaching the hidden platform (Fig. 1b), where aged mice required more than twice the time to reach the platform (22.3 ± 4.7 vs. 47.2 ± 5.9 s, respectively; P < 0.01). The difference in latency could not be attributed to differences in swim speed, which was similar between both cohorts (data not shown).
Within both groups, we observed a well-defined range of performance in learning and we next wanted to establish a standardized measure to quantify learning that would allow us to compare both young and aged animals. We defined the Learning Index as the relative improvement between the first and the last session, normalized to average performance of young animals using path length as quantitative measure. The Learning Index was 55.4 ± 10.9 % for young mice and 21.8 ± 12.1 % for aged mice (P < 0.01; Fig. 1c).
Decline in spatial learning and motor coordination with age. In young animals (6 months of age), performance in the swim maze test as assessed by path length (a) to the hidden platform improved significantly during the initial four learning sessions. In contrast, aged animals (24 months of age) did not improve during the first four sessions, and path length was significantly different from young animals during the fourth session. Swim speed did not differ between young and aged animals (b). The Learning Index, the relative improvement between the first and the last session, normalized to average performance of young animals was significantly lower in aged animals, compared with the young (c). In the bridge walking test, aged animals had significantly shorter latencies to fall in all trials (d), and average latency to fall was 35 % lower compared with young animals (e). Data are shown as mean ± SEM. Statistical significance is indicated, with *P < 0.05, **P < 0.01, and ***P < 0.001 as compared with young animals and ¶P < 0.05 and ¶¶ P < 0.01 as compared to session #1
Similarly, motor coordination was worse in aged animals compared with the young group, when tested using the bridge walking test. Performance on all four different bridges was significantly poorer in the aged group, compared with young mice (Fig. 1d). The average latency to fall across all bridges was defined as the index of performance. Average latency to fall was 49.7 ± 1.1 and 32.4 ± 1.4 s in young and aged mice, respectively (P < 0.001; Fig. 1e).
Homer-1a expression is decreased in the forebrain of aged mice
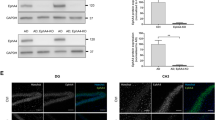
We next performed quantitative immunoblotting to measure the protein levels of Homer-1a and Homer-1b/c in the forebrain of the same mice 72 h after completing the behavioral assessment using GAPDH as loading control (Fig. 2a). The delay of 72 h was chosen to exclude transient effects of behavioral testing on the Homer-1 protein levels, particularly Homer-1a. Expression of the immediate early gene product Homer-1a was reduced by 71 % in the forebrain of aged mice (P < 0.001; Fig. 2b). In contrast, expression levels of Homer-1b/c did not differ significantly between young and aged mice. However, expression in aged mice showed a greater variability in the Homer-1b/c expression level (Fig. 2c); the coefficients of variance were 0.376 and 0.129 for aged and young mice, respectively. The reduction in Homer-1a protein expression resulted in a significant sixfold increase in the ratio of Homer-1b/c to Homer-1a from 11.5 ± 1.8 to 69.1 ± 20.3 in young and aged mice, respectively (P < 0.01; Fig. 2d). As GAPDH was used as endogenous control, we confirmed that absolute levels of GAPDH expression were similar between young and aged mice. The densities quantified from 12-bit images were 15,252 ± 821 and 14,517 ± 1,877 for young and aged mice, respectively, and were not statistically significantly different (P = 0.72). Furthermore, analysis without normalization for loading resulted in similar data for Homer-1a and Homer-1b/c expression levels.
Reduced Homer-1a expression in the aged forebrain. Quantitative immunoblotting was performed on individual forebrain samples of young and aged animals and the levels of Homer-1a and Homer-1b/c were quantified. GAPDH served as endogenous control (a). Homer-1a expression decreased by 71 % (b). In contrast, protein levels of Homer-1b/c did not differ between young and aged animals (c). The ratio of Homer-1b/c over Homer-1a was sixfold higher in aged animals, compared with the young cohort (d). Data are shown as mean ± SEM. Statistical significance is indicated as **P < 0.01 and ***P < 0.001 when compared with young animals
In order to validate this finding, we performed quantitative immunoblotting as well as quantitative polymerase chain reaction on a separate cohort of young and aged mice (Supplemental Fig. 1 and Supplemental methods). In this smaller cohort, we identified a 48 % reduction of Homer-1a mRNA levels in the forebrain that resulted in a 57 % reduction of Homer-1a protein levels. Homer-1c mRNA levels were similar between young and aged mice. In contrast, Homer-1b/c protein levels in the forebrain were elevated in aged mice; however, this difference did not reach statistical significance.
Homer-1a levels in the forebrain directly correlate with measures of spatial memory and motor coordination
Given the selective reduction in Homer-1a expression levels in aged animals, we next tested whether the level of Homer-1a in any given animal correlated with the animal’s performance in our behavioral tasks for spatial memory and learning (Fig. 3a–c) and motor coordination (Fig. 3d–f). We identified a highly statistically significant positive association between expression levels of Homer-1a and the Learning Index as a correlate for spatial learning (P < 0.001; Fig. 3a). Aged animals not only showed the lowest learning indices, but also had the lowest Homer-1a expression levels. The strength of this association had a Pearson’s coefficient of r = 0.687, corresponding to a coefficient of determination of r 2 = 0.472. In contrast, no association was found between the Learning Index and Homer-1b/c levels (P = 0.67; Fig. 3b). Consequently, the ratio of Homer-1b/c to Homer-1a expression had a statistically significant, negative correlation with the Learning Index (P < 0.05, r = 0.448, r 2 = 0.201; Fig. 3c).
Homer-1a levels correlate with behavioral measures for cognitive decline. Protein levels of Homer-1a correlated positively with a higher Learning Index, defined as the relative improvement between the first and the last session, normalized to average performance of young animals in the swim maze task (a). In contrast, no statistically significant association was found between Homer-1b/c expression and the Learning Index (b). The ratio of Homer-1b/c over Homer-1a was statistically significantly correlated with swim maze performance (c). When assessing associations between Homer-1 expression and performance in the bridge walking task, we found a strong positive correlation of Homer-1a with bridge walking (d), while expression levels of Homer-1b/c and the Homer-1b/c to Homer-1a ratio showed a trend towards a positive correlation with bridge walking performance (e, f). Empty circles in c and f represent the single outlier that was excluded from the association study by defining a difference of two standard deviations from the mean as the exclusion criterion
We next tested for association between Homer-1a expression levels and the average latency to fall as a correlate for motor coordination. Homer-1a showed a strong positive correlation with motor performance, evident as distinct clusters of young (green dots) and aged (red dots) animals in the correlation plot (P < 0.01, r = 0.603, r 2 = 0.364; Fig. 3d). Homer-1b/c expression showed a trend towards a negative correlation (P = 0.058; Fig. 3e), while the Homer-1b/c to Homer-1a ratio had a statistically significant negative correlation with the average latency to fall (P < 0.05, r = 0.553, r 2 = 0.306; Fig. 3f). A single data point (empty circle) was excluded from the analysis based on the mathematical outlier determination described in the “Analysis and statistics” section.
Discussion
Homer-1a prevents cognitive decline and loss of motor coordination with aging
In order to test the presence of an association between expression of synaptic proteins and correlates of spatial learning and motor coordination, we tested two cohorts of mice (6 and 24 months of age) in the swim maze and bridge walking tests, respectively. Both tests are well-established behavioral testing paradigms for the assessment of learning and motor coordination in rodents, and mice specifically (Collier and Coleman 1991). Our results are in agreement with the findings for mice of similar age published by us and others previously (Collier and Coleman 1991; Dubey et al. 1996; Forster et al. 1996; Sumien et al. 2004, 2006).
The cortex and hippocampus are the primary brain regions involved in spatial learning and memory and responsible for swim maze test performance of rodents (Dubey et al. 1996; Forster et al. 1996; Sumien et al. 2004, 2006; Nguyen 2006). Similarly, cortical and cerebellar networks are critical for motor learning and motor coordination (Hatsopoulos and Suminski 2011; Lamont and Weber 2012), as assessed in the present study by the bridge walking test. Detectable changes in synaptic proteins in the forebrain, therefore, are likely to directly impact spatial memory and learning as well as motor coordination. This hypothesis is further supported by the positive association of performance in the bridge walking task compared with the swim maze test (Supplemental Fig. 2). Our finding of a direct correlation of Homer-1a and the ratio of Homer-1b/c to Homer-1a with learning thus directly supports a role for Homer-1a in these processes. We observed a trend towards a significant negative correlation between Homer-1b/c expression in the forebrain and bridge walking performance indicating that potentially also an increase in Homer-1b/c expression with age partially contributes to the increase in the ratio of Homer-1b/c to Homer-1a.
Absence of Homer-1a protein in knockout mice resulted in water maze performance deficits (Jaubert et al. 2007), similar to those observed in the present study in the aged group. Furthermore, Homer-1a knockout mice have been shown to be critical for long-term fear memory formation (Inoue et al. 2009). In contrast, mice overexpressing Homer-1a selectively and inducibly in striatal medium spiny neurons showed deficits in motor learning and performance as well as drug-induced stereotypy (Tappe and Kuner 2006). A recent study investigated the expression levels of Homer proteins in the hippocampus during aging in Long–Evans rats (Menard and Quirion 2012). However, while this study aimed at measuring the effect of behavioral training on immediate early gene expression, our present study aimed at investigating non-induced expression levels of members of the Homer-1 protein family. Chronically reduced levels of Homer-1a during aging, as described in the present study, are thus likely contributors to the slow, gradual decline, while the loss of the ability to induce Homer-1a gene expression after behavioral training, as described in other studies, would contribute to the acute deficits in novel memory formation.
Immediate early genes and scaffolding proteins in aging
Other immediate early genes, such as c-fos (Rowe et al. 2007), and scaffolding proteins (Goh and Park 2009; Park and Reuter-Lorenz 2009; Proctor et al. 2010) have been implicated in the pathophysiology of aging and proposed to be a key regulatory mechanism in synaptic changes associated with age-related cognitive decline (Goh and Park 2009; Park and Reuter-Lorenz 2009). However, the involvement of Homer-1a offers a novel perspective on the mechanisms of progressive synaptic changing in aging: Homer-1a is encoded by an immediate early gene (Brakeman et al. 1997; Kato et al. 1998), while other splice variants encoded by the same gene are constitutively expressed scaffolding and signaling proteins (Brakeman et al. 1997; Kato et al. 1998; Duncan et al. 2005; Hayashi et al. 2009). Mechanistically, Homer-1a is thought to compete with Homer-1b/c for the physical interaction sites of the ligands of Homer-1b/c, providing a cellular mechanism for control of synaptic activity and endogenous neuroprotection (Duncan et al. 2005).
Virally mediated overexpression of Homer-1a in a rat seizure model attenuated seizures and protected neurons, while hippocampal-dependent memory was negatively impacted in these young animals (Klugmann et al. 2005). It would be interesting to study the effect of Homer-1 modulation on cognition in the aging central nervous system. Our study, in contrast, focuses on the physiological expression levels of Homer-1 isoforms in wild-type mice during normal aging.
Potential for pharmacological intervention targeting Homer-1a
Mechanistically, long Homer-1 isoforms serve as important scaffolding molecules that bring synaptic proteins into proximity (Duncan et al. 2005). For instance, coupling of inositol-1,4,5-trisphosphate receptors in the membranes of the endoplasmic reticulum and metabotropic glutamate receptors at the plasma membrane generates a structural and functional signaling domain (Tu et al. 1998; Mao et al. 2005; Menard and Quirion 2012) (Fig. 4). Furthermore, we have previously shown that Homer-1c directly modulates the biophysical properties of ryanodine receptors and that Homer-1a reverses these effects in living cells (Hwang et al. 2003; Westhoff et al. 2003; Duncan et al. 2005). The differential, cell type-specific expression levels and possible age-related changes of these Homer-1 binding partners will likely influence the effect of Homer-1a expression during aging. Future brain region-specific studies will help elucidate the underlying mechanism for this chronic loss of Homer-1a in the brains of cognitively impaired, aged mice. We herein describe for the first time a loss of baseline Homer-1a expression levels during aging and propose a novel cellular mechanism that in the future can be exploited pharmacologically, as supplementation of Homer-1a, Homer-1a analogues, or mimetics may prevent or delay cognitive decline in healthy aging.
Proposed mechanism of action. In the young brain, a homeostatic balance between Homer-1a and Homer-1b/c results in a normal equilibrium of synaptic proteins clustered by Homer-1b/c binding and thereby controls intracellular Ca2+ release. This results in the homeostasis of Ca2+ signaling and the expression of neuroprotective pathways, critical for normal CNS function, learning and memory formation as well as motor coordination. A loss of Homer-1a during aging results in increased coupling between plasma membrane G protein-coupled receptors (GPCR) and intracellular Ca2+ channels (ICC), leading to a net increase in intracellular Ca2+ release. This increased Ca2+ concentration resulting in Ca2+ toxicity and dyshomeostasis leading to the induction of neurodegenerative pathways and impairment of neuronal function
References
Aarts MM, Arundine M, Tymianski M (2003) Novel concepts in excitotoxic neurodegeneration after stroke. Expert Rev Mol Med 5:1–22
Baxter MG, Gallagher M (1996) Neurobiological substrates of behavioral decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging 17:491–495
Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF (1997) Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386:284–288
Brayton CF, Treuting PM, Ward JM (2012) Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol 49:85–105
Burroughs SL, Kaja S, Koulen P (2011) Quantification of deficits in spatial visual function of mouse models for glaucoma. Invest Ophthalmol Vis Sci 52:3654–3659
Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, Ingram DK, Jucker M (1998) Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging 19:599–606
Chen T, Fei F, Jiang XF, Zhang L, Qu Y, Huo K, Fei Z (2012) Down-regulation of Homer1b/c attenuates glutamate-mediated excitotoxicity through endoplasmic reticulum and mitochondria pathways in rat cortical neurons. Free Radic Biol Med 52:208–217
Collier TJ, Coleman PD (1991) Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging 12:685–693
Dubey A, Forster MJ, Lal H, Sohal RS (1996) Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys 333:189–197
Duncan RS, Hwang SY, Koulen P (2005) Effects of Vesl/Homer proteins on intracellular signaling. Exp Biol Med (Maywood) 230:527–535
Erickson CA, Barnes CA (2003) The neurobiology of memory changes in normal aging. Exp Gerontol 38:61–69
Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A 93:4765–4769
Gallagher M, Rapp PR (1997) The use of animal models to study the effects of aging on cognition. Annu Rev Psychol 48:339–370
Ghasemzadeh MB, Mueller C, Vasudevan P (2009) Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience 159:414–426
Giuffrida R, Musumeci S, D'Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV (2005) A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci 25:8908–8916
Goh JO, Park DC (2009) Neuroplasticity and cognitive aging: the scaffolding theory of aging and cognition. Restor Neurol Neurosci 27:391–403
Hatsopoulos NG, Suminski AJ (2011) Sensing with the motor cortex. Neuron 72:477–487
Haucke V, Neher E, Sigrist SJ (2011) Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci 12:127–138
Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y (2009) The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137:159–171
Hayflick L (2007) Biological aging is no longer an unsolved problem. Annals of the New York Academy of Sciences 1100:1–13
Hwang SY, Wei J, Westhoff JH, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P (2003) Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium 34:177–184
Ingram DK (1996) Brain-behavior linkages in aged rodent models: strategies for examining individual differences. Neurobiol Aging 17:497–499, discussion 500
Inoue N, Nakao H, Migishima R, Hino T, Matsui M, Hayashi F, Nakao K, Manabe T, Aiba A, Inokuchi K (2009) Requirement of the immediate early gene vesl-1S/homer-1a for fear memory formation. Mol Brain 2:7
Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF, Kang SH, Schwarz MK, Seeburg PH, Berman RF (2007) Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav 6:141–154
Kaja S, Duncan RS, Longoria S, Hilgenberg JD, Payne AJ, Desai NM, Parikh RA, Burroughs SL, Gregg EV, Goad DL, Koulen P (2011) Novel mechanism of increased Ca2+ release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors. Neuroscience 175:281–291
Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P (2003) Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci 44:3155–3162
Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K (1998) Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem 273:23969–23975
Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ (2005) AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci 28:347–360
Lamont MG, Weber JT (2012) The role of calcium in synaptic plasticity and motor learning in the cerebellar cortex. Neurosci Biobehav Rev 36:1153–1162
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ (2005) The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci 25:2741–2752
McDonald SR, Forster MJ (2005) Lifelong vitamin E intake retards age-associated decline of spatial learning ability in apoE-deficient mice. Age 27:5–16
Menard C, Quirion R (2012) Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS One 7:e28666
Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW (2012) Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 123:13–30
Nguyen PV (2006) Comparative plasticity of brain synapses in inbred mouse strains. J Exp Biol 209:2293–2303
Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y (2004) Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci 24:7648–7653
Park DC, Reuter-Lorenz P (2009) The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol 60:173–196
Proctor DT, Coulson EJ, Dodd PR (2010) Reduction in post-synaptic scaffolding PSD-95 and SAP-102 protein levels in the Alzheimer inferior temporal cortex is correlated with disease pathology. J Alzheimers Dis 21:795–811
Rapp PR, Amaral DG (1992) Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci 15:340–345
Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, Landfield PW (2007) Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci 27:3098–3110
Sumien N, Heinrich KR, Sohal RS, Forster MJ (2004) Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med 36:1424–1433
Sumien N, Sims MN, Taylor HJ, Forster MJ (2006) Profiling psychomotor and cognitive aging in four-way cross mice. Age 28:265–282
Tappe A, Kuner R (2006) Regulation of motor performance and striatal function by synaptic scaffolding proteins of the Homer1 family. Proc Natl Acad Sci U S A 103:774–779
Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF (1998) Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21:717–726
Westhoff JH, Hwang SY, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P (2003) Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell Calcium 34:261–269
Acknowledgments
Research reported in this publication was supported in part by the National Institute on Aging and the National Center for Research Resources of the National Institutes of Health under the award numbers P01AG022550, P01AG027956 (MJF, NS, PK), P01AG010485, S10RR022570 and S10RR027093 (PK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support for this study was provided by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, and the Vision Research Foundation of Kansas City (PK). The authors thank Jill Hilgenberg, Kathy Vernon and Amy Shah for excellent technical assistance and Margaret, Richard, and Sara Koulen for generous support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 855 kb)
About this article
Cite this article
Kaja, S., Sumien, N., Borden, P.K. et al. Homer-1a immediate early gene expression correlates with better cognitive performance in aging. AGE 35, 1799–1808 (2013). https://doi.org/10.1007/s11357-012-9479-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-012-9479-6