Abstract
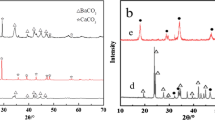
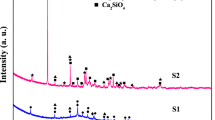
High-temperature solid adsorbent Li4SiO4 has received broad attention due to its high theoretical adsorption capacity, high regeneration capacity, and wide range of raw materials for preparation. In this paper, a Li4SiO4 adsorbent was prepared by MCM-48 as the silica precursor and modified by doping with metal ions (Ca2+ and Na+) for high-temperature capture of low-concentration CO2. The results showed that the surface of the Ca-doped (or Na-doped) Li4SiO4 adsorbent developed some particles that are primarily composed by Li2CaSiO4 (or Li3NaSiO4). Furthermore, the grains of the adsorbents became finer, effectively increasing the specific surface area and enhancing adsorption performance. Under 15 vol% CO2, the maximum CO2 adsorption was 25.63 wt% and 32.86 wt% when the Ca2+ doping amount was 0.06 and the Na+ doping amount was 0.12, respectively. These values were both higher than the adsorption capacity before the metal ion doping. After 10 adsorption/desorption cycles, the adsorption capacity of Na-doped Li4SiO4 increased by 9.68 wt%, while that of Ca-doped Li4SiO4 decreased by 7.98 wt%. This difference could be attributed to the easy sintering of the Ca-containing adsorbent. Furthermore, a biexponential model was used to fit the CO2 adsorption curve of the adsorbent in order to study the adsorption kinetics. Compared to the conventional Li4SiO4, the Ca/Na-doped adsorbent offers several advantages, such as a high CO2 adsorption capacity and stable cycling ability.







Similar content being viewed by others
Data availability
The authors do not have permission to share data.
References
Akeeb O, Wang L, Xie W, Davis R, Alkasrawi M, Toan S (2022) Post-combustion CO2 capture via a variety of temperature ranges and material adsorption process: a review. J Environ Manag 313:15026. https://doi.org/10.1016/j.jenvman.2022.115026
Avalos-Rendon T, Casa-Madrid J, Pfeiffer H (2009) Thermochemical capture of carbon dioxide on lithium aluminates (LiAlO2 and Li5AlO4): a new option for the CO2 absorption. J Phys Chem A 113(25):6919–6923. https://doi.org/10.1021/jp902501v
Bernabe-Pablo E, Plascencia-Hernandez F, Yanez-Aulestia A, Pfeiffer H (2020) High and efficient carbon dioxide chemisorption on a new high lithium-content ceramic; hexalithium cobaltate (Li6CoO4). Chem Eng J 384:123291. https://doi.org/10.1016/j.cej.2019.123291
Briz-Lopez EM, Ramirez-Moreno MJ, Romero-Ibarra IC, Gomez-Yanez C, Pfeiffer H, Ortiz-Landeros J (2016) First assessment of Li2OBi2O3 ceramic oxides for high temperature carbon dioxide capture. J Energy Chem 25(5):754–760. https://doi.org/10.1016/j.jechem.2016.05.001
Chang CL, Ilomaki J, Laurila H, McAleer M (2020) Causality between CO2 emissions and stock markets. Energies 13(11):2893. https://doi.org/10.3390/en13112893
Chen X, Xiong Z, Qin Y, Gong B, Tian C, Zhao Y, Zhang J, Zheng C (2016) High-temperature CO2 sorption by Ca-doped Li4SiO4 sorbents. Int J Hydrogen Energy 41(30):13077–13085. https://doi.org/10.1016/j.ijhydene.2016.05.267
Chen S, Qin C, Yuan W, Hanak DP, Ran J (2021) Kinetic study and modeling on the regeneration of Li4SiO4-based sorbents for high-temperature CO2 capture. Fuel Processing Technology 222:106976. https://doi.org/10.1016/j.fuproc.2021.106976
Chiron N, Guilet R, Deydier E (2003) Adsorption of Cu(II) and Pb(II) onto a grafted silica: isotherms and kinetic models. Water Environ Res 37(13):3079–3086. https://doi.org/10.1016/S0043-1354(03)00156-8
Cui H, Li X, Chen H, Gu X, Cheng Z, Zhou Z (2020) Sol-gel derived, Na/K-doped Li4SiO4-based CO2 sorbents with fast kinetics at high temperature. Chem Eng J 382:122807. https://doi.org/10.1016/j.cej.2019.122807
Fang Y, Zou R, Chen X (2020) High-temperature CO2 adsorption over Li4SiO4 sorbents derived from different lithium sources. Can J Chem Eng 98(7):1495–1500. https://doi.org/10.1002/cjce.23722
Fu R, Hu Y, Wang W (2023) Peering into the mechanisms of granulation and CO2 capture on alginate-assisted Li2CaSiO4-decorated Li4SiO4 sorbents. Chem Eng J 477:147156. https://doi.org/10.1016/j.cej.2023.147156
Gaultois MW, Dunstan MT, Bateson AW, Chan MSC, Grey CP (2018) Screening and characterization of ternary oxides for high-temperature carbon capture. Chem Mater 30(8):2535–2543. https://doi.org/10.1021/acs.chemmater.7b04679
Guo X, Ding L, Ren J, Yang H (2017) Preparation and CO2 capture properties of nanocrystalline Li2ZrO3 via an epoxide-mediated sol-gel process. J Sol-Gel Sci Technol 81(3):844–849. https://doi.org/10.1007/s10971-016-4233-7
Harada T, Hatton TA (2017) Tri-lithium borate (Li3BO3); a new highly regenerable high capacity CO2 adsorbent at intermediate temperature. J Materials Chem A 5(42):22224–22233. https://doi.org/10.1039/c7ta06167f
Hernandez-Palomares A, Alcantar-Vazquez B, Ramirez-Zamora RM, Coutino-Gonzalez E, Espejel-Ayala F (2023) CO2 capture using lithium-based sorbents prepared with constructionand demolition wastes as raw materials. Materials Today Sustainability 24:100491. https://doi.org/10.1016/j.mtsust.2023.100491
Hu Y, Lu H, Lv Zh, Zhang M, Yu G (2023) Pore reconstruction mechanism of wheat straw-templated Li4SiO4 pellets for CO2 capture. Sci Total Environ 856:159275. https://doi.org/10.1016/j.scitotenv.2022.159275
Kirikkaleli D, Sowah JK (2021) Time-frequency dependency of temperature and sea level: a global perspective. Environ Sci Pollut Res 28(41):58787–58798. https://doi.org/10.1007/s11356-021-14846-x
Pan Y, Zhang Y, Zhou T, Louis B, O’Hare D, Wang Q (2017) Fabrication of lithium silicates as highly efficient high-temperature CO2 sorbents from SBA-15 precursor. Inorg Chem 56(14):7821–7834. https://doi.org/10.1021/acs.inorgchem.7b00559
Quan S, Li SW, Xiao YC, Shao L (2017) CO2-selective mixed matrix membranes (MMMs) containing graphene oxide (GO) for enhancing sustainable CO2 capture. Int J Greenhouse Gas Control 56:22–29. https://doi.org/10.1016/j.ijggc.2016.11.010
Roman-Tejeda A, Pfeiffer H (2012) α→γ Lithium borate phase transition produced during the CO2 chemisorption process. J Therm Anal Calorim 110(2):807–811. https://doi.org/10.1007/s10973-011-1997-4
Ruan J, Tong Y, Ran J, Qin Ch (2023) Simplifying and optimizing Li4SiO4 preparation from spent LiFePO4 batteries with enhanced CO2. ACS Sustain Chem Eng 11(38):14158–14166. https://doi.org/10.1021/acssuschemeng.3c03695
Santos SCG, Garrido Pedrosa AM, Souza MJB, Cecilia JA, Rodriguez-Castellon E (2015) Carbon dioxide adsorption on micro-mesoporous composite materials of ZSM-12/MCM-48 type: the role of the contents of zeolite and functionalized amine. Mater Res Bull 70:663–672. https://doi.org/10.1016/j.materresbull.2015.05.037
Schwartz SE (2018) Resource letter GECC-1: the greenhouse effect and climate change: Earth’s natural greenhouse effect. Am J Phys 86(8):565–576. https://doi.org/10.1119/1.5045574
Shan S, Jia Q, Jiang L, Li Q, Wang Y, Peng J (2013) Novel Li4SiO4-based sorbents from diatomite for high temperature CO2 capture. Ceram Int 39(5):5437–5441. https://doi.org/10.1016/j.ceramint.2012.12.051
Shit SC, Shown I, Paul R, Chen KH, Mondal J, Chen LC (2020) Integrated nano-architectured photocatalysts for photochemical CO2 reduction. Nanoscale 12(46):23301–23332. https://doi.org/10.1039/d0nr05884j
Stefanelli E, Vitolo S, Puccini M (2022) Single-step fabrication of templated Li SiO -based pellets for CO capture at high temperature. Journal of Environmental Chemical Engineering 10(5):108389. https://doi.org/10.1016/j.jece.2022.108389
Togashi N, Okumura T, Oh-ishi K (2007) Synthesis and CO2 absorption property of Li4TiO4 as a novel CO2 absorbent. J Ceram Soc Jpn 115(1341):324–328. https://doi.org/10.2109/jcersj.115.324
Wang K, Yin Z, Zhao P, Zhou Z, Su Z, Ji S (2017) Development of metallic element-stabilized Li4SiO4 sorbents for cyclic CO2 capture. Int J Hydrogen Energy 42(7):4224–4232. https://doi.org/10.1016/j.ijhydene.2016.10.058
Wang K, Hong J, Zhou Z, Lin Z, Zhao P (2019) Development of alkali nitrate-containing Li4SiO4 for high-temperature CO2 capture. Energ Technol 7(2):325–332. https://doi.org/10.1002/ente.201800229
Wilczak A, Keinath TM (1993) Kinetics of sorption and desorption of copper(II) and lead(II) on activated carbon. Water Environ Res 65(3):238–244. https://doi.org/10.2175/WER.65.3.7
Yanez-Aulestia A, Pfeiffer H (2020) The role of nickel addition on the CO chemisorption enhancement in Ni-containing Li CuO : analysis of the cyclability and different CO partial pressure performance. Fuel 277:118185. https://doi.org/10.1016/j.fuel.2020.118185
Yang X, Liu W, Sun J, Hu Y, Wang W, Chen H, Zhang Y, Li X, Xu M (2016) Alkali-doped lithium orthosilicate sorbents for carbon dioxide capture. Chemsuschem 9(17):2480–2487. https://doi.org/10.1002/cssc.201600737
Zhang S, Chowdhury MBI, Zhang Q, de Lasa HI (2016) Novel fluidizable K-doped HAc-Li4SiO4 sorbent for CO2 capture preparation and characterization. Ind Eng Chem Res 55(49):12524–12531. https://doi.org/10.1021/acs.iecr.6b03746
Zhang Y, Gao Y, Louis B, Wang Q, Lin W (2019) Fabrication of lithium silicates from zeolite for CO2 capture at high temperatures. J Energy Chem 33:81–89. https://doi.org/10.1016/j.jechem.2018.08.014
Funding
This work was supported by the National Natural Science Foundation of China (No. 51966002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Dongling Zhao: writing — original draft, data curation. Linlin Geng: visualization, investigation. Yanfei Jia: conceptualization, methodology. Jianwen Wei: writing — review and editing, supervision. Xiaobin Zhou: software. Lei Liao: software.
Corresponding author
Ethics declarations
Ethical approval
We guarantee that our work is original and has not been submitted to other journals.
Consent to participate
All the authors listed have approved the manuscript.
Consent for publication
All authors have given their consent to publish the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, D., Geng, L., Jia, Y. et al. Adsorption of high-temperature CO2 by Ca2+/Na+-doped lithium orthosilicate: characterization, kinetics, and recycle. Environ Sci Pollut Res 31, 21267–21278 (2024). https://doi.org/10.1007/s11356-024-32252-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32252-x