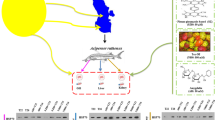
Abstract
The Caspian Sea has faced many environmental challenges, such as oil pollution. Heat shock proteins (HSPs) play a critical role in stress conditions and physiological changes caused by disease or injury. By evaluating the effects of various HSP inducers (HSPi), including Pro-Tex® (NOP: 800 mM), amygdalin (AMG: 80 mM), and a novel synthetic compound derived from pirano piranazole (SZ: 80 µm) on isolated cells from Sterlet Sturgeon (Acipenser ruthenus) treated with 75% IC50 PAH-benzo[a]pyrene (BaP; B75). This study examines whether there is a correlation between exposure to the BaP pollutant and HSPs in fish. In vitro, after culturing cells from the liver, kidney, and gills, they were treated with HSPi compounds in the presence and absence of BaP. Western blotting was used to assess HSP27, HSP70, and HSP90 expression patterns. A variety of enzyme activities were measured before (without treatment) and after treatment with HSPis and HSPi + B75, including cytochrome P450 (CYP450) activity, specific enzyme activity for acetylcholinesterase (AChE), antioxidant capacity, liver indicator enzymes, cortisol levels, and immunity parameters. When compared to the control group, cells treated with B75 showed the lowest AChE enzyme activity (p < 0.0001). CYP450 activity was highest in group B75, while HSPi caused the opposite effect (p < 0.0001). HSPi + B75 increased HSP levels and antioxidant parameters while decreasing cortisol and liver indicator enzymes (p < 0.0001). HSPi may be a powerful and reliable method for enhancing the resistance of A. ruthenus to BaP stresses before exposure. Treating cells with HSP-inducing compounds, such as NOP, AMG, and SZ, can assist them in managing stress and increase HSP (27, 70, and 90) protein expression. Furthermore, the study findings suggest that HSPis can also mitigate the adverse effects of stress, ultimately increasing cell survival and resistance.
Graphical Abstract







Similar content being viewed by others
Data availability
The data are available upon request.
References
Ahmadi K, Mirvaghefei AR, Banaee M, Vosoghei AR (2014) Effects of Long-Term Diazinon Exposure on Some Immunological and Haematological Parameters in Rainbow Trout Oncorhynchus Mykiss (Walbaum, 1792). Toxicol Environ Heal Sci 6(1):1–7
Akbary Moghaddam V et al (2021) A Novel Sulfamethoxazole Derivative as an Inhibitory Agent against HSP70: A Combination of Computational with in Vitro Studies. Int J Biol Macromol 189(July):194–205
Amani R, Heidari B, Ghafoori H, Valipour A (2022) Effects of silver nanocolloid and copper oxide (I) on HSP70 expression and GST activity in the Caspian Kutum. Turk J Fish Aquat Sci 22(6):TRJFAS19548
Baharloei M, Heidari B, Zamani H, Hadavi M (2020) Effects of Pro-Tex® on the Expression of Hsp70 Gene and Immune Response Parameters in the Persian Sturgeon Fingerlings, Acipenser Persicus, Infected with Aeromonas Hydrophila. J Appl Ichthyol 36(4):393–401
Bakhtyar S, Gagnon MM (2011) Comparison of Biomarker Responses Following One Dose of Benzo-a-Pyrene Administered to Three Native Australian Fish Species. J R Soc West Aust 94(3):465–472
Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2013) Biochemical and Histological Changes in the Liver Tissue of Rainbow Trout (Oncorhynchus Mykiss) Exposed to Sub-Lethal Concentrations of Diazinon. Fish Physiol Biochem 39(3):489–501
Baruah K et al (2014) Reactive Oxygen Species Generated by a Heat Shock Protein (Hsp) Inducing Product Contributes to Hsp70 Production and Hsp70-Mediated Protective Immunity in Artemia Franciscana against Pathogenic Vibrios. Dev Comp Immunol 46(2):470–479
Barulin NV (2015) Serum Enzyme Response of Captive Sturgeon Brookstock Acipenser Baerii Brandt 1869 Females and Two Hybrids (Bester=female Huso Huso Linnaeus, 1758×male Acipenser Ruthenus Linneaus, 1758, and RsSs=A.Gueldenstaedtii Brandt 1833×A.Baerii Brandt 1869) to Hormo. J Appl Ichthyol 31:2–6
Behera BK et al (2018) Polycyclic Aromatic Hydrocarbons (PAHs) in Inland Aquatic Ecosystems: Perils and Remedies through Biosensors and Bioremediation. Environ Pollut 241:212–233
Bolarinwa IF, Orfila C, Morgan MRA (2014) Amygdalin Content of Seeds, Kernels and Food Products Commercially- Available in the UK. Food Chem 152:133–139
Bradford MM (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem 72(1–2):248–254
Brough PA et al (2005) 3-(5-Chloro-2,4-Dihydroxyphenyl)-Pyrazole-4-Carboxamides as Inhibitors of the Hsp90 Molecular Chaperone. Bioorg Med Chem Lett 15(23):5197–5201
Butler M (2004) Animal cell culture and technology animal cell culture and technology. Taylor & Francis
Celi M, Vazzana M, Sanfratello MA, Parrinello N (2012) Elevated Cortisol Modulates Hsp70 and Hsp90 Gene Expression and Protein in Sea Bass Head Kidney and Isolated Leukocytes. Gen Comp Endocrinol 175(3):424–431
dos Santos DM et al (2014) Bioaccumulation of Butyltins and Liver Damage in the Demersal Fish Cathorops Spixii (Siluriformes, Ariidae). Environ Sci Pollut Res 21(4):3166–74
Ellman GL, Diane Courtney K, Andres V, Featherstone RM (1961) A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem Pharmacol 7(2):88–95
Esmaeilbeigi M et al (2021) Intra and Extracellular Effects of Benzo [α] Pyrene on Liver, Gill and Blood of Caspian White Fish (Rutilus Frissi Kutum): Cyto-Genotoxicity and Histopathology Approach. Mar Pollut Bull 163:111942
Focardi S et al (2000) Fish Cytochrome P4501A1 Activity Induced by Biobio River Sediments, South Central Chile. Bull Environ Contam Toxicol 65(2):175–182
Gao Z, Yao L, Pan L (2022) Gene Expression and Functional Analysis of Different Heat Shock Protein (HSPs) in Ruditapes Philippinarum under BaP Stress. Comp Biochem Physio Part - C: Toxicol Pharmacol 251:109194
Grela E, Kozłowska J, Grabowiecka A (2018) Current Methodology of MTT Assay in Bacteria – A Review. Acta Histochem 120(4):303–311
Hasanzadeh F et al (2021) Inhibition of NF-КB Expression in LPS-Induced RAW264.7 Macrophage Cells by a Thiazolidinone Derivative (TZDOCH 2 CH 3). Avicenna J Med Biochem 9(2):48–53
Holstein A, Kappas M, Propastin P, Renchin T (2018) Oil Spill Detection in the Kazakhstan Sector of the Caspian Sea with the Help of ENVISAT ASAR Data. Environ Earth Sci 77(5):1–11
Ikenaka Y et al (2013) Effects of Polycyclic Aromatic Hydrocarbons (PAHs) on an Aquatic Ecosystem: Acute Toxicity and Community-Level Toxic Impact Tests of Benzo[a]Pyrene Using Lake Zooplankton Community. J Toxicol Sci 38(1):131–136
Jahangirizadeh Z et al (2020) Rapid and Simple Screening of the Apoptotic Compounds Based on Hsp70 Inhibition Using Luciferase as an Intracellular Reporter. Anal Bioanal Chem 412(1):149–158
Jamalzadeh L et al (2016) Cytotoxic effects of some common organic solvents on MCF-7, RAW-264.7 and human umbilical vein endothelial cells. Avicenna J Med Biochem 4(1):e33453
Jiang J et al (2022) The toxic effects of combined exposure of chlorpyrifos and p, p’-DDE to zebrafish (Danio rerio) and tissue bioaccumulation. Aquat Toxicol 248:106194
Khoshbavar-Rostami Soltani M, Yelghi S, Hasanzati- Rostami A (2012) Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Water, Sediment and Tissue of Five Sturgeon Species in the Southern Caspian Sea Coastal Regions. Casp J Environ Sci 10(2):135–144
Kim JH, Kim SK, Hur YB (2019) Temperature-Mediated Changes in Stress Responses, Acetylcholinesterase, and Immune Responses of Juvenile Olive Flounder Paralichthys Olivaceus in a Bio-Floc Environment. Aquaculture 506(March):453–458
Küçükgüzel G, ŞenkardeŞ S (2015) Recent Advances in Bioactive Pyrazoles. Eur J Med Chem 97(1):786–815
Lakra WS, Raja Swaminathan T, Joy KP (2011) Development, Characterization, Conservation and Storage of Fish Cell Lines: A Review. Fish Physiol Biochem 37(1):1–20
Langan LM, Arossa S, Owen SF, Jha AN (2018) Assessing the Impact of Benzo[a]Pyrene with the in Vitro Fish Gut Model: An Integrated Approach for Eco-Genotoxicological Studies. Mutat Res - Genet Toxicol Environ Mutagen 826:53–64
Lattuada M, Albrecht C, Wilke T (2019) Differential Impact of Anthropogenic Pressures on Caspian Sea Ecoregions. Mar Pollut Bull 142:274–281
Lenhardt M, Jaric I, Kalauzi A, Cvijanovic G (2006) Assessment of Extinction Risk and Reasons for Decline in Sturgeon. Biodivers Conserv 15(6):1967–1976
Li ZH, Zhong LQ, Wei Na Mu, Yan Hua Wu (2016) Effects of Chronic Exposure to Tributyltin on Tissue-Specific Cytochrome P450 1 Regulation in Juvenile Common Carp. Xenobiotica 46(6):511–515
Li Q et al (2017) Effects of Dietary Lipid Sources on Growth Performance, Lipid Metabolism and Antioxidant Status of Juvenile Russian Sturgeon Acipenser Gueldenstaedtii. Aquac Nutr 23(3):500–510
Liu T, Pan L, Cai Y, Miao J (2015) Molecular Cloning and Sequence Analysis of Heat Shock Proteins 70 (HSP70) and 90 (HSP90) and Their Expression Analysis When Exposed to Benzo(a)Pyrene in the Clam Ruditapes Philippinarum. Gene 555(2):108–118
Long L et al (2019) Effects of Stocking Density on Growth, Stress, and Immune Responses of Juvenile Chinese Sturgeon (Acipenser Sinensis) in a Recirculating Aquaculture System. Comp Biochem Physiol Part - C: Toxicol Pharmacol 219:25–34
Moghim M, Kor D, Tavakolieshkalak M, Khoshghalb MB (2006) Stock Status of Persian Sturgeon (Acipenser Persicus Borodin, 1897) along the Iranian Coast of the Caspian Sea. J Appl Ichthyol 22(SUPPL. 1):99–107
Mrdaković M et al (2016) Acetylcholinesterase (AChE) and Heat Shock Proteins (Hsp70) of Gypsy Moth (Lymantria Dispar L.) Larvae in Response to Long-Term Fluoranthene Exposure. Chemosphere 159:565–569
Nichols J et al (2007) Use of in Vitro Absorption, Distribution, Metabolism, and Excretion (ADME) Data in Bioaccumulation Assessments for Fish. Hum Ecol Risk Assess 13(6):1164–1191
Olivares-Rubio HF, Espinosa-Aguirre JJ (2021) Acetylcholinesterase Activity in Fish Species Exposed to Crude Oil Hydrocarbons: A Review and New Perspectives. Chemosphere 264:128401
Oliveira HHP et al (2015) Mixtures of Benzo(a)Pyrene, Dichlorodiphenyltrichloroethane and Tributyltin Are More Toxic to Neotropical Fish Rhamdia Quelen than Isolated Exposures. Ecotoxicol Environ Saf 122:106–115
Otaka M et al (2007) The Induction Mechanism of the Molecular Chaperone HSP70 in the Gastric Mucosa by Geranylgeranylacetone (HSP-Inducer). Biochem Biophys Res Commun 353(2):399–404
Pacini N et al (2013) Antioxidant Response versus Selenium Accumulation in the Liver and Kidney of the Siberian Sturgeon (Acipenser Baeri). Chemosphere 93(10):2405–2412
Palanikumar L, Kumaraguru AK, Ramakritinan CM, Anand M (2012) Genotoxic Assessment of Anthracene and Benzo [a] Pyrene to Milkfish Chanos Chanos. Toxicol Environ Chem 94(2):350–363
Perrichon P et al (2014) Influence of Sediment Composition on PAH Toxicity Using Zebrafish (Danio Rerio) and Japanese Medaka (Oryzias Latipes) Embryo-Larval Assays. Environ Sci Pollut Res 21(24):13703–13719
Pirali M et al (2020) Artesunate, as a HSP70 ATPase Activity Inhibitor, Induces Apoptosis in Breast Cancer Cells. Int J Biol Macromol 164:3369–3375
Prokopchuk G, Dzyuba B, Rodina M, Cosson J (2016) Control of Sturgeon Sperm Motility: Antagonism between K+ Ions Concentration and Osmolality. Anim Reprod Sci 164:82–89
Rehberger K et al (2017) 20 Years of Fish Immunotoxicology-What We Know and Where We Are. Crit Rev Toxicol 47(6):509–535
Rey-Salgueiro L, Costa J, Ferreira M, Reis-Henriques MA (2011) Evaluation of 3-Hydroxy-Benzo[a]Pyrene Levels in Nile Tilapia (Oreochromis Niloticus) after Waterborne Exposure to Benzo[a]Pyrene. Toxicol Environ Chem 93(10):2040–2054
Roberts RJ et al (2010) Heat Shock Proteins (Chaperones) in Fish and Shellfish and Their Potential Role in Relation to Fish Health: A Review. J Fish Dis 33(10):789–801
Robertson JL, Jones MM, Olguin E, Alberts B (2017) Bioassays with arthropods, third edition bioassays with arthropods, 3rd edn. CRC. http://rguir.inflibnet.ac.in:8080/jspui/handle/123456789/10963
Sang W et al (2012) The Involvement of Heat Shock Protein and Cytochrome P450 Genes in Response to UV-A Exposure in the Beetle Tribolium Castaneum. J Insect Physiol 58(6):830–836
Seemann F et al (2015) Insight into the Transgenerational Effect of Benzo[a]Pyrene on Bone Formation in a Teleost Fish (Oryzias Latipes). Comp Biochem Physiol Part - C: Toxicol Pharmacol 178:60–67
Shahriyari-Nejad H et al (2020) Synthesis of Pirano-Based Piranazole-Based Compounds to Induce Apoptosis by Reducing the Expression of Anti-Apoptotic Protein B2 Cell Lymphoma Protein in the MCF-7 Human Breast Cancer Cell Category. Armaghane Danesh 25(1):40–54
Shirmohammadi M et al (2018) Using Cell Apoptosis, Micronuclei and Immune Alternations as Biomarkers of Phenanthrene Exposure in Yellowfin Seabream (Acanthopagrus Latus). Fish Shellfish Immunol 72:37–47
Soltani T, Safahieh A, Zolgharnain H, Matroodi S (2019) Interactions of Oxidative DNA Damage and CYP1A Gene Expression with the Liver Enzymes in Klunzinger’s Mullet Exposed to Benzo[a]Pyrene. Toxicol Rep 6:1097–1103
Stanic B et al (2006) Assessing Pollution in the Danube River near Novi Sad (Serbia) Using Several Biomarkers in Sterlet (Acipenser Ruthenus L.). Ecotoxicol Environ Saf 65(3):395–402
Taheri M et al (2022) Synthesis, in Vitro Biological Evaluation and Molecular Modelling of New 2-Chloro-3-Hydrazinopyrazine Derivatives as Potent Acetylcholinesterase Inhibitors on PC12 Cells. BMC Chem 16(1):1–12
Tarazi S et al (2021) Enhanced Soluble Expression of Glutathione S-Transferase Mu from Rutilus Kutum by Co-Expression with Hsp70 and Introducing a Novel Inhibitor for Its Activity. Process Biochem 111:261–266
Tresguerres M et al (2020) Evolutionary Links between Intra- and Extracellular Acid-Base Regulation in Fish and Other Aquatic Animals. J Exp Zool Part A: Ecol Integr Physiol 333(6):449–465
Vahdani F, Ghafouri H, Sarikhan S, Khodarahmi R (2019) Molecular Cloning, Expression, and Functional Characterization of 70-KDa Heat Shock Protein, DnaK, from Bacillus Halodurans. Int J Biol Macromol 137:151–159
Vahdatiraad L et al (2023a) Biological Responses of Stellate Sturgeon Fingerlings (Acipenser Stellatus) Immersed in HSP Inducer to Salinity Changes. Mar Environ Res 191:106145
Vahdatiraad L et al (2023b) Protective Effects of HSP Inducer on Diazinon-Exposed Stellate Sturgeon (Acipenser Stellatus) Fry: Insights on HSP70 Gene Expression, Immune Response, and Enzyme Indices. PLoS One 18(11):e0294188
Valipour A, Heidari B, Hadavi M, Yousefi A (2018) Changes in Immune Parameters (Lysozyme, IgM, C3) in Early Life Stages and Broodstock of Siberian Sturgeon, Acipenser Baerii. Fish Aquat Life 26(1):21–30
Vavrek MJ, Murray AM, Bell PR (2014) An Early Late Cretaceous (Cenomanian) Sturgeon (Acipenseriformes) from the Dunvegan Formation, Northwestern Alberta, Canada. Can J Earth Sci 51(7):677–681
Werner I, Linares-Casenave J, Van Eenennaam JP, Doroshov SI (2007) The Effect of Temperature Stress on Development and Heat-Shock Protein Expression in Larval Green Sturgeon (Acipenser Mirostris). Environ Biol Fishes 79(3–4):191–200
Woo SJ, Chung JK (2020) Cytochrome P450 1 Enzymes in Black Rockfish, Sebastes Schlegelii: Molecular Characterization and Expression Patterns after Exposure to Benzo[a]Pyrene. Aquat Toxicol 226:105566
Yan J, Wang L, Fu PP, Hongtao Yu (2004) Photomutagenicity of 16 Polycyclic Aromatic Hydrocarbons from the US EPA Priority Pollutant List. Mutat Res – Genet Toxicol Environ Mutagen 557(1):99–108
Yancheshmeh RA, Bakhtiari AR, Mortazavi S, Savabieasfahani M (2014) Sediment PAH: Contrasting Levels in the Caspian Sea and Anzali Wetland. Mar Pollut Bull 84(1–2):391–400
Yavar Ashayeri N et al (2018) Presence of Polycyclic Aromatic Hydrocarbons in Sediments and Surface Water from Shadegan Wetland – Iran: A Focus on Source Apportionment, Human and Ecological Risk Assessment and Sediment-Water Exchange. Ecotoxicol Environ Saf 148:1054–66
Yildirim NC, Yildirim N, Danabas D, Danabas S (2014) Use of Acetylcholinesterase, Glutathione S-Transferase and Cytochrome P450 1A1 in Capoeta Umbla as Biomarkers for Monitoring of Pollution in Uzuncayir Dam Lake (Tunceli, Turkey). Environ Toxicol Pharmacol 37(3):1169–1176
Zena R et al (2015) Exposure of Sea Bream (Sparus Aurata) to Toxic Concentrations of Benzo[a]Pyrene: Possible Human Health Effect. Ecotoxicol Environ Saf 122:116–125
Zhu F et al (2017) Benzo(a)Pyrene Degradation and Microbial Community Responses in Composted Soil. Environ Sci Pollut Res 24(6):5404–5414
Acknowledgements
We express our gratitude to Dr. Mohammed Ghadamyari for helping us determine the IC50 of BaP. Additionally, we are grateful to the Shahid Beheshti Sturgeon Breeding and Rearing Center (Rasht, Iran) for providing fish.
Funding
This work was supported by the Iranian National Science Foundation (INSF, Grant No. 97015418) and the University of Guilan. Furthermore, funds from the Caspian Sea Water Basin Research Institute grant have been utilized (Grant No. 961113100001).
Author information
Authors and Affiliations
Contributions
Sevda Zarei: Performing the experiments, Writing – original draft, Statistical Analysis. Hossein Ghafouri: Conceptualization, supervision, methodology, writing – review & editing. Leila Vahadatiraad: Performing the experiments. Behrooz Heidari: Methodology, Advisor, Writing – review & editing. Tooraj Sohrabi: Providing the sample and advice on fish physiology.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that they have no known competing financial interests or personal relationships that seem to affect the work reported in this article. We declare that we have no human participants, human data, or human tissues.
Consent for publication
All of the authors have read and approved the paper for publication.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The role of HSPs in the immune system and antioxidant systems is vital.
• Sterlet sturgeon cells were treated with HSP inducers with and without BaP.
• In response to HSPi and BaP, a significant increase in HSPs expression was observed.
• HSPi can modulate the antioxidant, liver indicator enzymes, AChE, and cytochrome P450 activities of Acipenser ruthenus against BaP.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zarei, S., Ghafouri, H., Vahdatiraad, L. et al. Enhancing resistance and cell survival in Acipenser ruthenus liver, gill, and kidney cells: The potential of heat shock protein inducers against PAH-benzo[a]pyrene stress. Environ Sci Pollut Res 31, 9445–9460 (2024). https://doi.org/10.1007/s11356-024-31884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-31884-3