Abstract
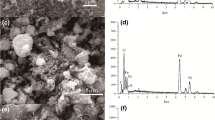
Heavy metal contamination has increased over the globe, causing significant environmental issues owing to direct and indirect releases into water bodies. As a result, metal removal from water entities must be addressed soon. Various adsorbents such as MOFs and chitosan have demonstrated promising results in water treatment. The present study prepared a composite material (chitosan-UiO-66-glycidyl methacrylate MOF) by a microwave-assisted method. The structure and morphology of the chitosan-MOF composite were studied using FE-SEM, EDX, XRD, BET, FT-IR, and TGA techniques. In addition, the adsorption of Pb(II) from aqueous solution onto the chitosan-MOF composite was analyzed in a batch study concerning pH, contact time, initial metal ion concentration, and adsorbent dosage. The composite has a large surface area of 867 m2/g with a total pore volume of 0.51 cm3/g and thermal stability of up to 400 \(^\circ \mathrm{C}\). Following an analysis of the adsorption isotherms, kinetics, and thermodynamics, the Langmuir model showed an excellent fit with the adsorption data (R2 = 0.99) and chi-squared (X2 = 3.609). The adsorption process was a spontaneous exothermic reaction and the pseudo-second-order rate equation fitted the kinetic profile well. Moreover, the composite is recyclable, retaining 83.45% of its removal effectiveness after 5 consecutive cycles, demonstrating it as a sustainable adsorbent for metal recovery. This study introduces a novel synthesized composite with enhanced recyclability and a higher potential for eliminating pollutants from industrial wastewater.















Similar content being viewed by others
Data availability
N/A.
References
Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, Atieh MA (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161
Abid HR, Shang J, Ang H-M, Wang S (2013) Amino-functionalized Zr-MOF nanoparticles for adsorption of CO2 and CH4. Int J Smart Nano Mater 4(1):72–82
Afshariazar F, Morsali A, Wang J, and Junk PC (2020) Highest and fastest removal rate of PbII ions through rational functionalized decoration of a metal–organic framework cavity. Chem–A Eur J 26(6) 1355–1362
Ahmadijokani F, Mohammadkhani R, Ahmadipouya S, Shokrgozar A, Rezakazemi M, Molavi H, . . . Arjmand M (2020) Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem Eng J 399:125346
Atia AA, Donia AM, Yousif AM (2005) Comparative study of the recovery of silver (I) from aqueous solutions with different chelating resins derived from glycidyl methacrylate. J Appl Polym Sci 97(3):806–812
Cao Y, Zhang H, Song F, Huang T, Ji J, Zhong Q, . . . Xu Q (2018) UiO-66-NH2/GO composite: synthesis, characterization and CO2 adsorption performance. Materials 11(4):589
Chen J, Sun B, Sun C, Zhang P, Xu W, Liu Y, . . . Tang K (2020) Immobilization of lipase AYS on UiO-66-NH2 metal-organic framework nanoparticles as a recyclable biocatalyst for ester hydrolysis and kinetic resolution. Sep Purif Technol 251:117398
Cheraghipour E, Pakshir M (2020) Process optimization and modeling of Pb (II) ions adsorption on chitosan-conjugated magnetite nano-biocomposite using response surface methodology. Chemosphere 260:127560
De Mesquita JP, Donnici CL, Teixeira IF, Pereira FV (2012) Bio-based nanocomposites obtained through covalent linkage between chitosan and cellulose nanocrystals. Carbohyd Polym 90(1):210–217
Deng Y-Y, Xiao X-F, Wang D, Han B, Gao Y, Xue J-L (2020) Adsorption of Cr (VI) from aqueous solution by ethylenediaminetetraacetic acid-chitosan-modified metal-organic framework. J Nanosci Nanotechnol 20(3):1660–1669
Dennis G, Harrison W, Agnes K, Erastus G (2016) Effect of biological control antagonists adsorbed on chitosan immobilized silica nanocomposite on Ralstonia solanacearum and growth of tomato seedlings. Adv Res 1–23
Dou XB, Chai MY, Zhu Y, Yang WT, Xu FJ (2013) Aminated poly (glycidyl methacrylate) s for constructing efficient gene carriers. ACS Appl Mater Interfaces 5(8):3212–3218
El-Maghrabi HH, Hosny R, Ramzi M, Fathy M (2017) Novel mesoporous silica (MCM-41) and its characterization for oil adsorption from produced water injected in water injection projects using fixed bed column processes. Desalin Water Treat 60:70–77
Elizalde-Peña E, Zarate-Triviño D, Nuño-Donlucas S, Medina-Torres L, Gough J, Sanchez I, . . . Luna-Barcenas G (2013) Synthesis and characterization of a hybrid (chitosan-g-glycidyl methacrylate)–xanthan hydrogel. J Biomater Sci Polym Ed 24(12):426-1442
Elwakeel KZ, Atia AA, Donia AM (2009) Removal of Mo (VI) as oxoanions from aqueous solutions using chemically modified magnetic chitosan resins. Hydrometallurgy 97(1–2):21–28
Elwakeel KZ, El-Bindary AA, Kouta EY (2017) Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell. J Environ Chem Eng 5(4):3698–3710
Elwakeel KZ, El-Bindary AA, Kouta EY, Guibal E (2018) Functionalization of polyacrylonitrile/Na-Y-zeolite composite with amidoxime groups for the sorption of Cu (II), Cd (II) and Pb (II) metal ions. Chem Eng J 332:727–736
Fiaz M, Athar M (2020) Enhancing the hydrogen and oxygen evolution reaction efficiency of amine functionalized MOF NH2-UiO-66 via incorporation of CuO nanoparticles. Catal Lett 150(11):3314–3326
Ghosh D, Saha R, Ghosh A, Nandi R, Saha B (2015) A review on toxic cadmium biosorption from contaminated wastewater. Desalin Water Treat 53(2):413–420
Govindan S, Nivethaa EAK, Saravanan R, Narayanan V, Stephen A (2012) Synthesis and characterization of chitosan–silver nanocomposite. Appl Nanosci 2(3):299–303
Gul Zaman H, Baloo L, Pendyala R (2022) Application in the optimization of Pb (II) adsorption by chitosan from produced water by using response surface methodology. Int J Environ Sci Technol 1–12
Hall JN, Bollini P (2019) Structure, characterization, and catalytic properties of open-metal sites in metal organic frameworks. React Chem Eng 4(2):207–222
Hasankola ZS, Rahimi R, Shayegan H, Moradi E, Safarifard V (2020) Removal of Hg2+ heavy metal ion using a highly stable mesoporous porphyrinic zirconium metal-organic framework. Inorg Chim Acta 501:119264
He HJ, Xiang ZH, Chen XJ, Chen H, Huang H, Wen M, Yang CP (2018) Biosorption of Cd (II) from synthetic wastewater using dry biofilms from biotrickling filters. Int J Environ Sci Technol 15(7):1491–1500
Huang A, Wan L, Caro J (2018) Microwave-assisted synthesis of well-shaped UiO-66-NH2 with high CO2 adsorption capacity. Mater Res Bull 98:308–313
Jamshidifard S, Koushkbaghi S, Hosseini S, Rezaei S, Karamipour A, Irani M (2019) Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb (II), Cd (II) and Cr (VI) ions from aqueous solutions. J Hazard Mater 368:10–20
Jia X, Zhang B, Chen C, Fu X, Huang Q (2021) Immobilization of chitosan grafted carboxylic Zr-MOF to porous starch for sulfanilamide adsorption. Carbohyd Polym 253:117305
Khan T, Mustafa MRU, Isa MH, Abd Manan TSB, Ho Y-C, Lim J-W, Yusof NZ (2017) Artificial neural network (ANN) for modelling adsorption of lead (Pb (II)) from aqueous solution. Water Air Soil Pollut 228(11):1–15
Khataee A, Alidokht L, Hassani A, Karaca S (2013) Response surface analysis of removal of a textile dye by a Turkish coal powder. Adv Environ Res 2:291–308
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231
Lim S-F, Lee AYW (2015) Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ Sci Pollut Res 22(13):10144–10158
Mahmoud ME, Amira MF, Seleim SM, Mohamed AK (2020) Amino-decorated magnetic metal-organic framework as a potential novel platform for selective removal of chromium (Vl), cadmium (II) and lead (II). J Hazard Mater 381:120979
Mansouri M, Sadeghian S, Mansouri G, Setareshenas N (2021) Enhanced photocatalytic performance of UiO-66-NH2/TiO2 composite for dye degradation. Environ Sci Pollut Res 28(20):25552–25565
Mashabi RA, Khan ZA, Elwakeel KZ (2022) Chitosan or glycidyl methacrylate-based adsorbents for dyes removal from the aqueous solutions: a brief review. Mater Adv
Molavi H, Eskandari A, Shojaei A, Mousavi SA (2018a) Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66 (Zr). Microporous Mesoporous Mater 257:193–201
Molavi H, Joukani FA, Shojaei A (2018b) Ethylenediamine grafting to functionalized NH2–UiO-66 using green aza-michael addition reaction to improve CO2/CH4 adsorption selectivity. Ind Eng Chem Res 57(20):7030–7039
Molavi H, Moghimi H, Taheri RA (2020) Zr-based MOFs with high drug loading for adsorption removal of anti-cancer drugs: a potential drug storage. Appl Organomet Chem 34(4):e5549
Molavi H, Shojaei A, Mousavi SA (2018c) Improving mixed-matrix membrane performance via PMMA grafting from functionalized NH 2–UiO-66. J Mater Chem A 6(6):2775–2791
Molavi H, Shojaei A, Pourghaderi A (2018d) Rapid and tunable selective adsorption of dyes using thermally oxidized nanodiamond. J Colloid Interface Sci 524:52–64
Naeimi S, Faghihian H (2017) Performance of novel adsorbent prepared by magnetic metal-organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Sep Purif Technol 175:255–265
Nik OG, Chen XY, Kaliaguine S (2012) Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J Membr Sci 413:48–61
Patrulea V, Negrulescu A, Mincea MM, Pitulice LD, Spiridon OB, Ostafe V (2013) Optimization of the removal of copper (II) ions from aqueous solution on chitosan and cross-linked chitosan beads. BioResources 8(1):1147–1165
Rakati KK, Mirzaei M, Maghsoodi S, Shahbazi A (2019) Preparation and characterization of poly aniline modified chitosan embedded with ZnO-Fe3O4 for Cu (II) removal from aqueous solution. Int J Biol Macromol 130:1025–1045
Rallapalli P, Prasanth KP, Patil D, Somani RS, Jasra RV, Bajaj HC (2011) Sorption studies of CO 2, CH 4, N 2, CO, O 2 and Ar on nanoporous aluminum terephthalate [MIL-53 (Al)]. J Porous Mater 18(2):205–210
Ramezanzadeh M, Ramezanzadeh B, Bahlakeh G, Tati A, Mahdavian M (2021) Development of an active/barrier bi-functional anti-corrosion system based on the epoxy nanocomposite loaded with highly-coordinated functionalized zirconium-based nanoporous metal-organic framework (Zr-MOF). Chem Eng J 408:127361
Rezakazemi M, Amooghin AE, Montazer-Rahmati MM, Ismail AF, Matsuura T (2014) State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog Polym Sci 39(5):817–861
Roushani M, Saedi Z, Baghelani YM (2017) Removal of cadmium ions from aqueous solutions using TMU-16-NH2 metal organic framework. Environ Nanotechnol Monit Manag 7:89–96
Saleem H, Rafique U, Davies RP (2016) Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater 221:238–244
Saleh TA (2016) Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb (II): from surface properties to sorption mechanism. Desalin Water Treat 57(23):10730–10744
Shayegan H, Ali GA, Safarifard V (2020) Amide-functionalized metal–organic framework for high efficiency and fast removal of Pb (II) from aqueous solution. J Inorg Organomet Polym Mater 30(8):3170–3178
Solis KLB, Kwon Y-H, Kim M-H, An H-R, Jeon C, Hong Y (2020) Metal organic framework UiO-66 and activated carbon composite sorbent for the concurrent adsorption of cationic and anionic metals. Chemosphere 238:124656
Tian P, He X, Li W, Zhao L, Fang W, Chen H, . . . Wang W (2018) Zr-MOFs based on Keggin-type polyoxometalates for photocatalytic hydrogen production. J Mater Sci 53(17):12016-12029
Tu Y-J, You C-F, Chen M-H, Duan Y-P (2017) Efficient removal/recovery of Pb onto environmentally friendly fabricated copper ferrite nanoparticles. J Taiwan Inst Chem Eng 71:197–205
Wang K, Gu J, Yin N (2017) Efficient removal of Pb (II) and Cd (II) using NH2-functionalized Zr-MOFs via rapid microwave-promoted synthesis. Ind Eng Chem Res 56(7):1880–1887
Wang K, Tao X, Xu J, Yin N (2016) Novel chitosan–MOF composite adsorbent for the removal of heavy metal ions. Chem Lett 45(12):1365–1368
Wang L, Xing R, Liu S, Yu H, Qin Y, Li K, . . . Li P (2010) Recovery of silver (I) using a thiourea-modified chitosan resin. J Hazard Mater 180(1-3):577-582
Wu S, Ge Y, Wang Y, Chen X, Li F, Xuan H, Li X (2018) Adsorption of Cr (VI) on nano Uio-66-NH2 MOFs in water. Environ Technol 39(15):1937–1948
Wu Y-N, Zhou M, Zhang B, Wu B, Li J, Qiao J, . . . Li F (2014) Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 6(2):1105-1112
Xiong C, Wang S, Zhang L, Li Y, Zhou Y, Peng J (2018) Selective recovery of silver from aqueous solutions by poly (glycidyl methacrylate) microsphere modified with trithiocyanuric acid. J Mol Liq 254:340–348
Yang Z, Tong X, Feng J, He S, Fu M, Niu X, . . . Feng X (2019) Flower-like BiOBr/UiO-66-NH2 nanosphere with improved photocatalytic property for norfloxacin removal. Chemosphere 220:98-106
Yin N, Wang K, Li Z (2016) Rapid microwave-promoted synthesis of Zr-MOFs: an efficient adsorbent for Pb (II) removal. Chem Lett 45(6):625–627
Yin XC, Liu X, Fan JC, Wu JJ, Men JL, Zheng GS (2017) Preparation of gel resins and removal of copper and lead from water. J Appl Polym Sci 134(7)
Younas M, Rezakazemi M, Daud M, Wazir MB, Ahmad S, Ullah N, Ramakrishna S (2020) Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog Energy Combust Sci 80:100849
Yuan S, Zhang J, Yang Z, Tang S, Liang B, Pehkonen SO (2017) Click functionalization of poly (glycidyl methacrylate) microspheres with triazole-4-carboxylic acid for the effective adsorption of Pb (II) ions. New J Chem 41(14):6475–6488
Zarate-Triviño DG, Pool H, Vergara-Castañeda H, Elizalde-Peña EA, Vallejo-Becerra V, Villaseñor F, . . . Luna-Barcenas G (2020) (Chitosan-g-glycidyl methacrylate)-collagen II scaffold for cartilage regeneration. Int J Polym Mater Polym Biomater 69(16):1043-1053
Zhang X, Yan L, Li J, Yu H (2020) Adsorption of heavy metals by l-cysteine intercalated layered double hydroxide: kinetic, isothermal and mechanistic studies. J Colloid Interface Sci 562:149–158
Zhao F, Yang W, Han Y, Luo X, Tang W, Yue T, Li Z (2021) A straightforward strategy to synthesize supramolecular amorphous zirconium metal-organic gel for efficient Pb (II) removal. Chem Eng J 407:126744
Zheng Y, Rao F, Zhang M, Li J, Huang W (2021) Efficient, selective, and reusable metal–organic framework-based adsorbent for the removal of Pb (II) and Cr (VI) heavy-metal pollutants from wastewater. Clean Eng Technol 5:100344
Zhu J, Wu L, Bu Z, Jie S, Li B-G (2019) Polyethyleneimine-modified UiO-66-NH2 (Zr) metal–organic frameworks: preparation and enhanced CO2 selective adsorption. ACS Omega 4(2):3188–3197
Funding
The authors were financially supported by Universiti Teknologi PETRONAS via the YUTP grant with cost center 015LCO-303.
Author information
Authors and Affiliations
Contributions
Conceptualization, H.G.Z. and L.B.; supervision, L.B, and S.R.M.K and F.A. Writing—original draft preparation, H.G.Z.; writing—review and editing, M.A., A.A, and K.A; funding acquisition, L.B. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
N/A
Consent to participate
N/A
Consent for publication
N/A
Competing interests
The authors declare no competing interests to disclose.
Additional information
Responsible Editor: George Z. Kyzas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gul Zaman, H., Baloo, L., Kutty, S.R. et al. Insight into microwave-assisted synthesis of the chitosan-MOF composite: Pb(II) adsorption. Environ Sci Pollut Res 30, 6216–6233 (2023). https://doi.org/10.1007/s11356-022-22438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-22438-6