Abstract
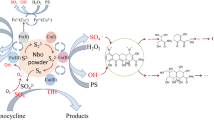
This study evaluated the improvement of bisphenol A (BPA) elimination through hydrogen sulfite (HS) coupling with persulfate (PS) activated by low amounts of Fe2+. Under the optimum condition (10 μM Fe2+, 0.6 mM HS, 0.8 mM PS, pH = 4.0), 100% BPA (5 μM) was removed within 15 min. Sulfate radical (SO4•−) and singlet oxygen (1O2) were confirmed as the primary active species for BPA degradation in the Fe2+/HS/PS system, and the steady-state concentration of SO4•− and 1O2 was 2.43 × 10−9 M and 1.67 × 10−9 M, respectively. Besides, FeHSO3+ and FeOHSO3H+ were the main iron species in the Fe2+/HS/PS system. The removal potency of BPA depended on the operation parameters, such as chemical reagent dosages, reaction temperature, and the solution initial pH. The impact of NO3−, SO42−, and humic acid (HA) on BPA removal was negligible, whereas Cl−, HCO3−, and HPO42− restrained BPA decomposition. Two injections of HS could improve the limitation of BPA degradation efficiency due to the rapid consumption of HS in the reaction process. The lower removal efficiency of BPA was observed in real water matrices than that in ultrapure water. Whatever, up to 58.1%, 66.3%, 68.1%, and 88.1% of BPA were removed from domestic wastewater, lake water, river water, and tap water within 10 min, respectively. In addition, the BPA degradation process was characterized by the 3D fluorescence spectra technique, which indicated the BPA oxidation intermediates also have fluorescence characteristics. Moreover, 6 intermediate products were identified, and the possible degradation pathways of BPA were proposed. Additionally, the Fe2+/HS/PS system also exerted an excellent performance for the removal of other representative organic contaminants including enrofloxacin, acid orange 7, acetaminophen, and phenol. All results indicated that the Fe2+/HS/PS system could be a promising method for organic pollutant removal.










Similar content being viewed by others
Data availability
The authors declare that all relevant data supporting the findings of this study are included in this article and its supplementary information files.
References
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J Chem Phys 17:513–886. https://doi.org/10.1063/1.555805
Bu L, Zhou S, Zhou SQ (2016) Modeling of Fe(II)-activated persulfate oxidation using atrazine as a target contaminant. Sep Purif Technol 169:59–65. https://doi.org/10.1016/j.seppur.2016.05.037
Careghini A, Mastorgio AF, Saponaro S, Sezenna E (2015) Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res 22:5711–5741. https://doi.org/10.1007/s11356-014-3974-5
Chaves MJS, Barbosa SC, Primel EG (2021) Emerging contaminants in Brazilian aquatic environment: identifying targets of potential concern based on occurrence and ecological risk. Environ Sci Pollut Res 28:67528–67543. https://doi.org/10.1007/s11356-021-15245-y
Chen J, Qian Y, Liu H, Huang T (2016) Oxidative degradation of diclofenac by thermally activated persulfate: implication for ISCO. Environ Sci Pollut Res 23:3824–3833. https://doi.org/10.1007/s11356-015-5630-0
Chen L, Peng X, Liu J, Li J, Wu F (2012) Decolorization of orange II in aqueous solution by an Fe (II)/sulfite system: replacement of persulfate. Ind Eng Chem Res 51:13632–13638. https://doi.org/10.1021/ie3020389
Chen M, Zhang Z, Zhu L, Wang N, Tang H (2019a) Bisulfite-induced drastic enhancement of bisphenol A degradation in Fe3+-H2O2 Fenton system. Chem Eng J 369:1190–1197. https://doi.org/10.1016/j.cej.2018.12.170
Chen X, Hu X, Gao Y (2019b) Removal of NO in simulated flue with aqueous solution of peroxymonosulfate activated by high temperature and Fe (II). Chem Eng J 359:419–427. https://doi.org/10.1016/j.cej.2018.10.198
Chen Y, Ouyang D, Zhang W, Yan J, Qian L, Han L, Chen M (2020) Degradation of benzene derivatives in the CuMgFe-LDO/persulfate system: the role of the interaction between the catalyst and target pollutants. J Environ Sci 90:87–97. https://doi.org/10.1016/j.jes.2019.11.014
Clifton CL, Huie RE (1989) Rate constants for hydrogen abstraction reactions of the sulfate radical, SO4− Alcohols. Int J Chem Kinet 21:677–687. https://doi.org/10.1002/kin.550210807
Deng Y, Yan C, Nie M, Ding M (2021) Bisphenol A adsorption behavior on soil and biochar: impact of dissolved organic matter. Environ Sci Pollut Res 28:32434–32445. https://doi.org/10.1007/s11356-021-12723-1
Dong H, Chen J, Feng L, Zhang W, Guan X, Strathmann TJ (2019) Degradation of organic contaminants through activating bisulfite by cerium (IV): a sulfate radical-predominant oxidation process. Chem Eng J 357:328–336. https://doi.org/10.1016/j.cej.2018.09.024
Du J, Guo W, Wang H, Yin R, Zheng H, Feng X, Che D, Ren N (2018) Hydroxyl radical dominated degradation of aquatic sulfamethoxazole by Fe(0)/bisulfite/O2: kinetics, mechanisms, and pathways. Water Res 138:323–332. https://doi.org/10.1016/j.watres.2017.12.046
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for 493 degradation of environmental organic pollutants: review. Chem Eng J 310:41–62. https://doi.org/10.1016/j.cej.2016.10.064
Haag WR, Gassman E (1984) Singlet oxygen in surface waters—Part I: Furfuryl alcohol as a trapping agent. Chemosphere 13:631–640. https://doi.org/10.1016/0045-6535(84)90199-1
Jiang B, Liu Y, Zheng J, Tan M, Wang Z, Wu M (2015) Synergetic transformations of multiple pollutants driven by Cr (VI)–sulfite reactions. Environ Sci Technol 49:12363–12371. https://doi.org/10.1021/acs.est.5b03275
Jiang X, Wu Y, Wang P, Li H, Dong W (2013) Degradation of bisphenol A in aqueous solution by persulfate activated with ferrous ion. Environ Sci Pollut Res 20:4947–4953. https://doi.org/10.1007/s11356-013-1468-5
Lei Y, Zhang H, Wang J, Ai J (2015) Rapid and continuous oxidation of organic contaminants with ascorbic acid and a modified ferric/persulfate system. Chem Eng J 270:73–79. https://doi.org/10.1016/j.cej.2015.02.014
Li X, Wu B, Zhang Q, Liu Y, Wang J, Li F, Ma F, Gu Q (2020) Complexation of humic acid with Fe ions upon persulfate/ferrous oxidation: further insight from spectral analysis. J Hazard Mater 399:123071. https://doi.org/10.1016/j.jhazmat.2020.123071
Liu Z, Guo Y, Shang R, Fang Z, Wu F, Wang Z (2016) A triple system of Fe (III)/sulfite/persulfate: decolorization and mineralization of reactive Brilliant Red X-3B in aqueous solution at near-neutral pH values. J Taiwan Inst Chem E 68:162–168. https://doi.org/10.1016/j.jtice.2016.08.027
Lutze HV, Bircher S, Rapp I, Kerlin N, Bakkour R, Geisler M, von Sonntag C, Schmidt TC (2015) Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environ Sci Technol 49:1673–1680. https://doi.org/10.1021/es503496u
Lu Z, Cao X, Wei H, Huo W, Wang Q, Li K (2021) Strong enhancement effect of bisulfite on MIL-68(Fe)-catalyzed Fenton-like reaction for organic pollutants degradation. Appl Surf Sci 542:148631. https://doi.org/10.1016/j.apsusc.2020.148631
Neta P, Huie RE, Ross AB (1988) Rate constants for reactions of inorganic radicals in aqueous solution. J Phys Chem Ref Data 17:1027–1284. https://doi.org/10.1063/1.555808
Nie M, Yan C, Li M, Wang X, Bi W, Dong W (2015) Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem Eng J 279:507–515. https://doi.org/10.1016/j.cej.2015.05.055
Palharim PH, Graça CAL, Teixeira ACSC (2020) Comparison between UVA-and zero-valent iron-activated persulfate processes for degrading propylparaben. Environ Sci Pollut Res 27:22214–22224. https://doi.org/10.1007/s11356-020-08141-4
Petrie B, Lopardo L, Proctor K, Youdan J, Barden R, Kasprzyk-Hordern B (2019) Assessment of bisphenol-A in the urban water cycle. Sci Total Environ 650:900–907. https://doi.org/10.1016/j.scitotenv.2018.09.011
Qi C, Liu X, Li Y, Lin C, Ma J, Li X, Zhang H (2017) Enhanced degradation of organic contaminants in water by peroxydisulfate coupled with bisulfite. J Hazard Mater 328:98–107. https://doi.org/10.1016/j.jhazmat.2017.01.010
Romero A, Santos A, Vicente F, González C (2010) Diuron abatement using activated persulphate: effect of pH, Fe (II) and oxidant dosage. Chem Eng J 162:257–265. https://doi.org/10.1016/j.cej.2010.05.044
Roshani B, vel NK (2011) The influence of persulfate addition for the degradation of micropollutants by ionizing radiation. Chem Eng J 168:784-789. https://doi.org/10.1016/j.cej.2010.12.023
Sun P, Zhang K K, Zhang Y (2020) Sunflower-straw-derived biochar-enhanced Fe (III)/S2O82- system for degradation of benzoic acid. Huan Jing ke Xue 41(5): 2301–2309. https://doi.org/10.13227/j.hjkx.201908187
Varanasi L, Coscarelli E, Khaksari M, Mazzoleni LR, Minakata D (2018) Transformations of dissolved organic matter induced by UV photolysis, hydroxyl radicals, chlorine radicals, and sulfate radicals in aqueous-phase UV-based advanced oxidation processes. Water Res 135:22–30. https://doi.org/10.1016/j.watres.2018.02.015
Wang C, Zhao Z, Deng X, Chen R, Liang J, Shi W, Cui F (2021a) Ultrafast oxidation of emerging contaminants by novel VUV/Fe2+/PS process at wide pH range: performance and mechanism. Chem Eng J 426:131921. https://doi.org/10.1016/j.cej.2021.131921
Wang H, Deng J, Lu X, Wan L, Huang J, Liu Y (2021b) Rapid and continuous degradation of diclofenac by Fe (II)-activated persulfate combined with bisulfite. Sep Purif Technol 262:118335. https://doi.org/10.1016/j.seppur.2021.118335
Wu L, Lin Y, Zhang Y, Wang P, Ding M, Nie M, Yan C, Chen S (2021a) Ca (OH)2-mediated activation of peroxymonosulfate for the degradation of bisphenol S. RSC Adv 11:33626–33636. https://doi.org/10.1039/D1RA05286A
Wu S, Li H, Li X, He H, Yang C (2018) Performances and mechanisms of efficient degradation of atrazine using peroxymonosulfate and ferrate as oxidants. Chem Eng J 353:533–541. https://doi.org/10.1016/j.cej.2018.06.133
Wu S, Shen L, Lin Y, Yin K, Yang C (2021b) Sulfite-based advanced oxidation and reduction processes for water treatment. Chem Eng J 414:128872. https://doi.org/10.1016/j.cej.2021.128872
Wu S, Yang C, Lin Y, Cheng J (2022) Efficient degradation of tetracycline by singlet oxygen-dominated peroxymonosulfate activation with magnetic nitrogen-doped porous carbon. J Environ Sci (china) 115:330–340. https://doi.org/10.1016/j.jes.2021.08.002
Xie P, Guo Y, Chen Y, Wang Z, Shang R, Wang S, Ding J, Wan Y, Jiang W, Ma J (2017) Application of a novel advanced oxidation process using sulfite and zero-valent iron in treatment of organic pollutants. Chem Eng J 314:240–248. https://doi.org/10.1016/j.cej.2016.12.094
Xie P, Zhang L, Chen J, Ding J, Wan Y, Wang S, Wang Z, Zhou A, Ma J (2019) Enhanced degradation of organic contaminants by zero-valent iron/sulfite process under simulated sunlight irradiation. Water Res 149:169–178. https://doi.org/10.1016/j.watres.2018.10.078
Xu J, Ding W, Wu F, Mailhot G, Zhou D, Hanna K (2016) Rapid catalytic oxidation of arsenite to arsenate in an iron(III)/sulfite system under visible light. Appl Catal B Environ 186:56–61. https://doi.org/10.1016/j.apcatb.2015.12.033
Yin R, Hu L, Xia D, Yang J, He C, Liao Y, Zhang Q, He J (2020) Hydroxylamine promoted Fe(III)/Fe(II) cycle on ilmenite surface to enhance persulfate catalytic activation and aqueous pharmaceutical ibuprofen degradation. Catal Today 358:294–302. https://doi.org/10.1016/j.cattod.2019.04.081
Yu Y, Li S, Peng X, Yang S, Zhu Y, Chen L, Wu F, Mailhot G (2016) Efficient oxidation of bisphenol A with oxysulfur radicals generated by iron-catalyzed autoxidation of sulfite at circumneutral pH under UV irradiation. Environ Chem Lett 14:527–532. https://doi.org/10.1007/s10311-016-0573-3
Zhang W, Zhou S, Sun J, Meng X, Luo J, Zhou D, Crittenden J (2018) Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ Sci Technol 52:7380–7389. https://doi.org/10.1021/acs.est.8b01662
Zhang X, Ding Y, Tang H, Han X, Zhu L, Wang N (2014) Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: efficiency, stability and mechanism. Chem Eng J 236:251–262. https://doi.org/10.1016/j.cej.2013.09.051
Zhang Y, Lou J, Wu L, Nie M, Yan C, Ding M, Wang P, Zhang H (2021) Minute Cu2+ coupling with HCO3− for efficient degradation of acetaminophen via H2O2 activation. Ecotox Environ Safe 221:112422. https://doi.org/10.1016/j.ecoenv.2021.112422
Zhang Y, Nie S, Nie M, Yan C, Qiu L, Wu L, Ding M (2022a) Remediation of sulfathiazole contaminated soil by peroxymonosulfate: performance, mechanism and phytotoxicity. Sci Total Environ 830:154839. https://doi.org/10.1016/j.scitotenv.2022.154839
Zhang Y, Zhou J, Li C, Guo S, Wang G (2012) Reaction kinetics and mechanism of iron(II)-induced catalytic oxidation of sulfur(IV) during wet desulfurization. Ind Eng Chem Res 51:1158–1165. https://doi.org/10.1021/ie2014372
Zhang Z, Du C, Zhang Y, Yu G, Xiong Y, Zhou L, Liu Y, et al (2022b) Degradation of oxytetracycline by magnetic MOFs heterojunction photocatalyst with persulfate: high stability and wide range. Environ Sci Pollut Res 1-11. https://doi.org/10.1007/s11356-021-17971-9
Zhen G, Lu X, Li Y, Zhao Y, Wang B, Song Y, Chai X, Niu D, Cao X (2012) Novel insights into enhanced dewaterability of waste activated sludge by Fe (II)-activated persulfate oxidation. Bioresour Technol 119(9):7–14. https://doi.org/10.1016/j.biortech.2012.05.115
Zhou D, Yuan Y, Yang S, Gao H, Chen L (2015) Roles of oxysulfur radicals in the oxidation of acid orange 7 in the Fe(III)–sulfite system. J Sulfur Chem 36:373–384. https://doi.org/10.1080/17415993.2015.1028939
Ziajka J, Beer F, Warneck P (1994) Iron-catalysed oxidation of bisulphite aqueous solution: evidence for a free radical chain mechanism. Atmos Environ 28:2549–2552. https://doi.org/10.1016/1352-2310(94)90405-7
Zou J, Ma J, Chen L, Li X, Guan Y, Xie P, Pan C (2013) Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ Sci Technol 47:11685–11691. https://doi.org/10.1021/es4019145
Funding
This research was financially supported by the National Natural Science Foundation of China (42067034 and 42067058), the Training Project for Major Academic Disciplines and Technology Leader of Jiangxi Province (20212BCJL23058), the Jiangxi Provincial Natural Science Foundation (20202BAB203015 and 20202BAB203014), and the Open Fund of Key Laboratory of Eco-geochemistry, Ministry of Natural Resources (ZSDHJJ202004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. MN and CY designed all experiments; chemical analysis, experimental data collection, and data curation were performed by SC, MN, CY, LW, MD, and PW; the first draft of the manuscript was written by SC, MN, and CY. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work did not report on or involve the use of any animal or human data or tissue.
Consent for publication
This work did not contain data from any individual person.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Yan, C., Nie, M. et al. Hydrogen sulfite promoted the activation of persulfate by μM Fe2+ for bisphenol A degradation. Environ Sci Pollut Res 29, 85185–85201 (2022). https://doi.org/10.1007/s11356-022-21801-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21801-x