Abstract
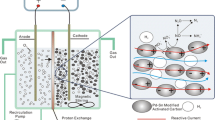
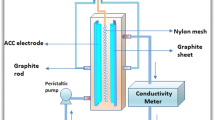
In this paper, nitric acid-modified activated carbon was used as an electrode in the electrosorption process for the removal of Co2+, Mn2+, and Ni2+ from wastewater. The effects of applied voltage, initial pH, and coexisting ions on removal efficiency were investigated. The adsorption process was evaluated by adsorption isotherm models. The results indicated that the electrosorption process was consistent with the Langmuir model, proving that the electrosorption process was a monolayer adsorption process. The maximum adsorption capacities of Co2+, Mn2+, and Ni2+ were 131.58 mg/g, 102.04 mg/g, and 103.09 mg/g. Electrochemical tests revealed that the specific capacitance of AC-HNO3 was 54.11 F/g when the scanning rate was 5 mV/s, while the specific capacitance of AC was 36.51 F/g. The Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) confirmed that the content of oxygen groups on the surface of activated carbon increased after modification, which provided more adsorption sites for electrosorption. When the selected concentration of HCl was used as the eluent, the elution efficiency of Co2+, Mn2+, and Ni2+ could reach 94.23%, 93.65%, and 90.61%. The removal efficiency could reach more than 95% after three cycles. The results of the study can be used as a reference significance for the removal of cobalt, manganese, and nickel ions from heavy metal wastewater by electrosorption.


















Similar content being viewed by others
Data availability
The authors can confirm that all relevant data are included in the article.
References
Abdullah N, Yusof N, Lau WJ, Jaafar J, Ismail AF (2019) Recent trends of heavy metal removal from water/wastewater by membrane technologies. J Ind Eng Chem 76:17–38
Agartan L, Akuzum B, Mathis T, Ergenekon K, Agar E, Caglan Kumbur E (2018) Influence of thermal treatment conditions on capacitive deionization performance and charge efficiency of carbon electrodes. Sep Purif Technol 202:67–75
An Q, Zhang C, Zhao B, Li Z, Deng S, Wang T, Jin L (2021) Insight into synergies between Acinetobacter sp. AL-6 and pomelo peel biochar in a hybrid process for highly efficient manganese removal. Sci Total Environ 793:148609
Anand Raj M, Arumainathan S (2019) Comparative study of hydrogen evolution behavior of nickel cobalt and nickel cobalt magnesium alloy film prepared by pulsed electrodeposition. Vacuum 160:461–466
Angeles AT, Lee J (2021) Carbon-based capacitive deionization electrodes: development techniques and its influence on electrode properties. Chem Rec 21:820–840
Baby R, Saifullah B, Hussein MZ (2019) Palm kernel shell as an effective adsorbent for the treatment of heavy metal contaminated water. Sci Rep 9:18955
Boudrahem F, Aissani-Benissad F, Soualah A (2011) Adsorption of lead(II) from aqueous solution by using leaves of date trees as an adsorbent. J Chem Eng Data 56:1804–1812
Carvajal-Flórez E, Santiago-Alonso C-G (2019) Technologies applicable to the removal of heavy metals from landfill leachate. Environ Sci Pollut Res 26:15725–15753
Chai WS, Cheun JY, Kumar PS, Mubashir M, Majeed Z, Banat F, Ho S-H, Show PL (2021) A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J Clean Prod 296:126589
Chang L, Hu YH (2018) Highly conductive porous Na-embedded carbon nanowalls for high-performance capacitive deionization. J Phys Chem Solids 116:347–352
Chen Y, Zhu Y, Wang Z, Li Y, Wang L, Ding L, Gao X, Ma Y, Guo Y (2011) Application studies of activated carbon derived from rice husks produced by chemical-thermal process—a review. Adv Coll Interface Sci 163:39–52
Choi JY, Choi JH (2010) A carbon electrode fabricated using a poly(vinylidene fluoride) binder controlled the Faradaic reaction of carbon powder. J Ind Eng Chem 16:401–405
Cojocaru C, Zakrzewska-Trznadel G, Jaworska A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. part 1: Optimization of complexation conditions. J Hazard Mater 169:599–609
El-Hendawy A-NA (2003) Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon. Carbon 41:713–722
Esposito A, Pagnanelli F, Vegliò F (2002) pH-related equilibria models for biosorption in single metal systems. Chem Eng Sci 57:307–313
Foo KY, Hameed BH (2009) A short review of activated carbon assisted electrosorption process: an overview, current stage and future prospects. J Hazard Mater 170:552–559
Gabelich CJ, Tran TD, Suffet IHM (2002) Electrosorption of inorganic salts from aqueous solution using carbon aerogels. Environ Sci Technol 36:3010–3019
Gaikwad MS, Balomajumder C (2017) Simultaneous electrosorptive removal of chromium(VI) and fluoride ions by capacitive deionization (CDI): Multicomponent isotherm modeling and kinetic study. Sep Purif Technol 186:272–281
He M, Wang L, Lv Y, Wang X, Zhu J, Zhang Y, Liu T (2020) Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem Eng J 389:124452
Hezarjaribi M, Bakeri G, Sillanpää M, Chaichi MJ, Akbari S, Rahimpour A (2021) New strategy to enhance heavy metal ions removal from synthetic wastewater by mercapto-functionalized hydrous manganese oxide via adsorption and membrane separation. Environ Sci Pollut Res 28:51808–51825
Hou C, Huang C (2013) A comparative study of electrosorption selectivity of ions by activated carbon electrodes in capacitive deionization. Desalination 314:124–129
Huang S, Fan C, Hou C (2014a) Electro-enhanced removal of copper ions from aqueous solutions by capacitive deionization. J Hazard Mater 278:8–15
Huang W, Zhang Y, Bao S, Cruz R, Song S (2014b) Desalination by capacitive deionization process using nitric acid-modified activated carbon as the electrodes. Desalination 340:67–72
Huang Z, Lu L, Cai Z, Ren ZJ (2016) Individual and competitive removal of heavy metals using capacitive deionization. J Hazard Mater 302:323–331
Huang Z, Yang Z, Kang F, Inagaki M (2017) Carbon electrodes for capacitive deionization. J Mater Chem A 5:470–496
Kalfa A, Shapira B, Shopin A, Cohen I, Avraham E, Aurbach D (2020) Capacitive deionization for wastewater treatment: opportunities and challenges. Chemosphere 241:125003
Kurniawan TA, Chan GYS, Lo W-h, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366:409–426
Lang J, Yan X, Liu W, Wang R, Xue Q (2012) Influence of nitric acid modification of ordered mesoporous carbon materials on their capacitive performances in different aqueous electrolytes. J Power Sources 204:220–229
Li P, Gui Y, Blackwood DJ (2018a) Development of a nanostructured α-MnO2/carbon paper composite for removal of Ni2+/Mn2+ ions by electrosorption. ACS Appl Mater Interfaces 10:19615–19625
Li R, Li L, Zhang Z, Chen G, Tang Y (2021) Limiting factors of heavy metals removal during anaerobic biological pretreatment of municipal solid waste landfill leachate. J Hazard Mater 416:126081
Li Y, Stewart TC, Tang HL (2018b) A comparative study on electrosorptive rates of metal ions in capacitive deionization. J Water Process Eng 26:257–263
Liu X, Wu J, Liu C, Wang J (2017) Removal of cobalt ions from aqueous solution by forward osmosis. Sep Purif Technol 177:8–20
Liu X, Wu J, Wang J (2019) Electro-enhanced removal of cobalt ions from aqueous solution by capacitive deionization. Sci Total Environ 697:134144
Lu J, Dreisinger D, Glück T (2014) Manganese electrodeposition — a literature review. Hydrometallurgy 141:105–116
Luciano MA, Ribeiro H, Bruch GE, Silva GG (2020) Efficiency of capacitive deionization using carbon materials based electrodes for water desalination. J Electroanal Chem 859:113840
Mangun CL, Benak KR, Daley MA, Economy J (1999) Oxidation of activated carbon fibers: effect on pore size, surface chemistry, and adsorption properties. Chem Mater 11:3476–3483
Mohammadzadeh Pakdel P, Peighambardoust SJ (2018) Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohyd Polym 201:264–279
Niu R, Li H, Ma Y, He L, Li J (2015) An insight into the improved capacitive deionization performance of activated carbon treated by sulfuric acid. Electrochim Acta 176:755–762
Oladunni J, Zain JH, Hai A, Banat F, Bharath G, Alhseinat E (2018) A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: from theory to practice. Sep Purif Technol 207:291–320
Patil DS, Chavan SM, Oubagaranadin JUK (2016) A review of technologies for manganese removal from wastewaters. J Environ Chem Eng 4:468–487
Qasem NAA, Mohammed RH, Lawal DU (2021) Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean Water 4:36
Qin H, Hu T, Zhai Y, Lu N, Aliyeva J (2020) The improved methods of heavy metals removal by biosorbents: a review. Environ Pollut 258:113777
Sarma GK, Sen Gupta S, Bhattacharyya KG (2019) Nanomaterials as versatile adsorbents for heavy metal ions in water: a review. Environ Sci Pollut Res 26:6245–6278
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452:301–310
Shrestha R, Ban S, Devkota S, Sharma S, Joshi R, Tiwari AP, Kim HY, Joshi MK (2021) Technological trends in heavy metals removal from industrial wastewater: a review. J Environ Chem Eng 9:105688
Soo KW, Wong KC, Goh PS, Ismail AF, Othman N (2020) Efficient heavy metal removal by thin film nanocomposite forward osmosis membrane modified with geometrically different bimetallic oxide. J Water Process Eng 38:101591
Sufiani O, Tanaka H, Teshima K, Machunda RL, Jande YAC (2020a) Enhanced electrosorption capacity of activated carbon electrodes for deionized water production through capacitive deionization. Sep Purif Technol 247:116998
Sufiani O, Tanaka H, Teshima K, Machunda RL, Jande YAC (2020b): Enhanced electrosorption capacity of activated carbon electrodes for deionized water production through capacitive deionization. Sep Purif Technol 247
Upadhyay U, Sreedhar I, Singh SA, Patel CM, Anitha KL (2021) Recent advances in heavy metal removal by chitosan based adsorbents. Carbohyd Polym 251:117000
Wang C, Li T, Yu G, Deng S (2021) Removal of low concentrations of nickel ions in electroplating wastewater using capacitive deionization technology. Chemosphere 284:131341
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang Q, Yu J, Chen X, Du D, Wu R, Qu G, Guo X, Jia H, Wang T (2019) Non-thermal plasma oxidation of Cu(II)-EDTA and simultaneous Cu(II) elimination by chemical precipitation. J Environ Manage 248:109237
Wu B, Weng X, Wang N, Yin M, Zhang L, An Q (2021) Chlorine-resistant positively charged polyamide nanofiltration membranes for heavy metal ions removal. Sep Purif Technol 275:119264
Xing M, Xu L, Wang J (2016) Mechanism of Co(II) adsorption by zero valent iron/graphene nanocomposite. J Hazard Mater 301:286–296
Xing W, Liang J, Tang W, He D, Yan M, Wang X, Luo Y, Tang N, Huang M (2020) Versatile applications of capacitive deionization (CDI)-based technologies. Desalination 482:114390
Xue Y, Cao M, Gao J, Gui Y, Chen J, Liu P, Ma F, Yan Y, Qiu M (2021) Electroadsorption of uranium on amidoxime modified graphite felt. Sep Purif Technol 255:117753
Yao S, Zhang J, Shen D, Xiao R, Gu S, Zhao M, Liang J (2016) Removal of Pb(II) from water by the activated carbon modified by nitric acid under microwave heating. J Colloid Interface Sci 463:118–127
Yu S, Xiong X, Ma J, Qian H (2021) One-step preparation of cobalt nickel oxide hydroxide@cobalt sulfide heterostructure film on Ni foam through hydrothermal electrodeposition for supercapacitors. Surf Coat Technol 426:127791
Yu X, Zhang J, Yan C, Shen X (2014) Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res 133:232–238
Zhang M, Lan G, Qiu H, Zhang T, Li W, Hu X (2019) Preparation of ion exchange resin using soluble starch and acrylamide by graft polymerization and hydrolysis. Environ Sci Pollut Res 26:3803–3813
Zhao C, Ge R, Zhen Y, Wang Y, Li Z, Shi Y, Chen X (2019) A hybrid process of coprecipitation-induced crystallization-capacitive deionization-ion exchange process for heavy metals removal from hypersaline ternary precursor wastewater. Chem Eng J 378:122136
Zhao Y, Hu X, Jiang B, Li L (2014) Optimization of the operational parameters for desalination with response surface methodology during a capacitive deionization process. Desalination 336:64–71
Zhu T, Lu Y, Zheng S, Chen Y, Guo H (2015) Influence of nitric acid activation on structure and capacitive performances of ordered mesoporous carbon. Electrochim Acta 152:456–463
Funding
This work was financially supported by the Yantai Science and technology planning project (2021MSGY029), the University and Local Integration Development Project of Yantai (2020XDRHXMPT36), the Academic-Industry Partnership Fund(210F0401006), and the National Natural Science Foundation of China: 21976047. The authors are grateful for all your support.
Author information
Authors and Affiliations
Contributions
All authors (Yun Xue, Wanting Cheng, Meng Cao, Jianzhang Gao, Jiaqi Chen, Yunyang Gui, Wenmin Zhu, and Fuqiu Ma) were equal contributors in writing this review article. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Weiming Zhang
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, Y., Cheng, W., Cao, M. et al. Development of nitric acid-modified activated carbon electrode for removal of Co2+/Mn2+/Ni2+ by electrosorption. Environ Sci Pollut Res 29, 77536–77552 (2022). https://doi.org/10.1007/s11356-022-21272-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21272-0