Abstract
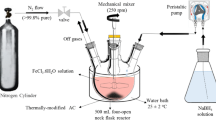
Improving the removal rate of pentavalent antimony (Sb(V)) by electrocoagulation (EC) is of great significance to the environment. In this paper, the EC with composite scrap iron and manganese filings as an anode (Fe–Mn EC) was investigated for the high-efficiency elimination of Sb(V). The results showed that Fe–Mn EC can enhance the removal of Sb(V) by 11.18–17.36% compared with the traditional iron electrocoagulation (Fe EC). Meanwhile, Sb(V) removal increased with the growth of current concentration as well as Mn content in the anode. However, the Sb(V) removal rate was inhibited when Mn content exceeded 20%. Moreover, the flocs generated during the Fe and Fe–Mn EC (Fe flocs and Fe–Mn flocs) were analyzed both structurally and theoretically using XRD, SEM, BET, and adsorption experiment. The results indicated that the components of Fe–Mn flocs were mostly Mn-substituted FeOOH, which appeared as the structure of nanometer flakes and large internal surface areas. Meanwhile, the Fe–Mn flocs had the ability of much faster Sb(V) adsorption rate; its Sb(V) adsorption capacity was 2.5 times more than that of the Fe flocs. The thermodynamics constants of both Fe and Fe–Mn flocs proved that adsorption was associated with monolayer physical adsorption. To the best of our knowledge, this is the first report of the electrocoagulation with composite scrap iron-manganese as an anode to remove Sb, which provide a new idea and potential technical support for the removal of Sb(V).








Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ainsworth CC, Girvin DC, Zachara JM, Smith SC (1989) Chromate Adsorption on goethite: effects of aluminum substitution. Soil Sci Soc Am J 53:411–418
Anderson PR, Benjamin MM (1985) Effect of silicon on the crystallization and adsorption properties of ferric oxides. Environ Sci Technol 19:1048
Ayub S, Siddique AA, Khursheed MS, Zarei A, Changani F (2020) Removal of heavy metals (Cr, Cu and Zn) from electroplating wastewater by electrocoagulation and adsorption processes. Desalin Water Treat 179:263–271
Bashir A, Malik LA, Ahad S, Manzoor T, Bhat MA, Dar GN, Pandith AH (2019) Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ Chem Lett 17(2):729–754
Bullough F, Weiss DJ, Dubbin WE, Coles BJ, Barrott J, Sengupta AK (2010) Evidence of competitive adsorption of Sb(III) and As(III) on activated alumina. Ind Eng Chem Res 49: 2521–2524
Cao D, Zeng H, Yang B, Zhao X (2017) Mn assisted electrochemical generation of two-dimensional Fe-Mn layered double hydroxides for efficient Sb(V) removal. J Hazard Mater 336:33–40
Chang F, Qu J, Liu H, Liu R, Zhao X (2009) Fe-Mn binary oxide incorporated into diatomite as an adsorbent for arsenite removal: preparation and evaluation. J Colloid Interface Sci 338(2):353–358
Charlier P, Abdallah FB, Bruneau R, Jacqueline S, Rasmussen KL (2017) Did the Romans die of antimony poisoning? The case of a Pompeii water pipe (79 CE). Toxicol Lett 281:184–186
Chen JJ, Zhang GP, Li HX, Fu ZP, Wu J (2015) Treatment of wastewater containing antimony by electrochemical hydride generation and recovery of antimony. Environ Sci 036(004):1338–1344 ((In Chinese))
Du H, Tao J, Yang R, Lei M, Tie B, Nie N, Liu X, Hu M, Xu Z (2015) Bacteria affect Sb(III, V) adsorption and oxidation on birnessite. J Soils Sediments 20:2418–2425
Fei Y, Zhang B, He J, Chen C, Liu H (2022) Dynamics of vertical vanadium migration in soil and interactions with indigenous microorganisms adjacent to tailing reservoir. J Hazard Mater 424:127608
Fil BA, Yilmaz AE, Boncukcuoğlu R, Bayar S (2012) Removal of divalent heavy metal ions from aqueous solution by Dowex HCR-S synthetic resin. Bul Chem Commun 44:201–207
Guan W, Zhang B, Tian S, Zhao X (2018) The synergism between electro-Fenton and electrocoagulation process to remove Cu-EDTA. Appl Catal B 227:252–257
Guo W, Fu Z, Hao W, Liu S, Wu F, Giesy JP (2018) Removal of antimonate (Sb(V)) and antimonite (Sb(III)) from aqueous solutions by coagulation-flocculation-sedimentation (CFS): Dependence on influencing factors and insights into removal mechanisms. Sci Total Environ 644:1277–1285
He M, Wang X, Wu F, Fu Z (2012) Antimony pollution in China. Sci Total Environ 421:41–50
Jiang HR, Shyy W, Wu MC, Zhang RH, Zhao TS (2019) A bi-porous graphite felt electrode with enhanced surface area and catalytic activity for vanadium redox flow batteries. Appl Energy 233:105–113
Khatibikamal V, Torabian A, Janpoor F, Hoshyaripour G (2010) Fluoride removal from industrial wastewater using electrocoagulation and its adsorption kinetics. J Hazard Mater 179(1–3):276–280
Kim SJ, Lim KH, Joo KH, Lee MJ, Kil SG (2002) Removal of heavy metal-cyanide complexes by ion exchange. Korean J Chem Eng 19(6):1078–1084
Kong X, Zhou Y, Xu T, Hu B, Lei X, Chen H, Yu G (2020) A novel technique of COD removal from electroplating wastewater by Fenton-alternating current electrocoagulation. Environ Sci Pollut Res 27:15198–15210
Kong Z, Song Y, Shao Z, Chai H (2021) Biochar-pyrite bi-layer bioretention system for dissolved nutrient treatment and by-product generation control under various stormwater conditions. Water Res 206: 117737
Long X, Wang X, Guo X, He M (2020) A review of removal technology for antimony in aqueous solution. J Environ Sci 90:189–204
Ozdemir N, Soylak M, Elci L, Dogan M (2004) Speciation analysis of inorganic Sb(III) and Sb(V) ions by using mini column filled with Amberlite XAD-8 resin. Analytica Chimica Acta 505: 37-41
Navarro P, Alguacil FJ (2002) Adsorption of antimony and arsenic from a copper electrorefining solution onto activated carbon. Hydrometallurgy 66(1–3):101–105
P SRD, Joshi SW, Lali AM, Gantayet LM, Verma R (2020) Anion exchange separation of antimony and the integrated ion exchange process for decontamination of used zircaloy pressure tubes from Indian pressurized heavy water reactors. Separation Science and Technology 55(13): 2325-2333
Sandoval MA, Fuentes R, Thiam A, Salazar R (2020) Arsenic and fluoride removal by electrocoagulation process: a general review. Sci Total Environ 753:142108
Sarı A, Çıtak D, Tuzen M (2010) Equilibrium, thermodynamic and kinetic studies on adsorption of Sb(III) from aqueous solution using low-cost natural diatomite. Chem Eng J 162:521–527
Scheinost AC, Stanjek H, Schulze DG, Gasser U, Sparks DL (2001) Structural environment and oxidation state of Mn in goethite-groutite solid-solutions. Am Miner 86(1–2):139–146
Schwertmann U, Cornell RM (2008) Iron oxides in the laboratory: preparation and characterization. John Wiley & Sons
Song P, Song Q, Yang Z, Zeng G, Xu H, Li X, Xiong W (2018) Numerical simulation and exploration of electrocoagulation process for arsenic and antimony removal: Electric field, flow field, and mass transfer studies. J Environ Manage 228:336–345
Song P, Yang Z, Xu H, Huang J, Yang X, Wang L (2014) Investigation of influencing factors and mechanism of antimony and arsenic removal by EC using Fe–Al electrodes. Ind Eng Chem Res 53(33):12911–12919
Sun X, Doner HE, Zavarin M (2018) Spectroscopy study of arsenite [As (III)] oxidation on Mn-substituted goethite. Clays Clay Miner 47(4):474–480
Tang X, Zheng H, Teng H, Sun Y, Guo J, Xie W, Yang Q, Chen W (2016) Chemical coagulation process for the removal of heavy metals from water: a review. Desalin Water Treat 57:1733–1748
Tegladza ID, Xu Q, Xu K, Lv G, Lu J (2020) Electrocoagulation processes: a general review about role of electro-generated flocs in pollutant removal. Process Saf Environ Prot 146:169–189
Tian Y, Liu G, Gao Y, Wang Y, Zhang J, Fang Y, Deng H (2021) Comparative study on As (III) and As (V) adsorption by CO32–intercalated Fe/Mn-LDHs from aqueous solution. Blue-Green Systems 3(1):175–190
Ungureanu G, Santos S, Rui B, Botelho C (2015) Arsenic and antimony in water and wastewater: overview of removal techniques with special reference to latest advances in adsorption-ScienceDirect. J Environ Manage 151:326–342
Wang Y, Gao Y, Zhu Z, Zhang L, Zhao N, Fang Y, Liu G (2021) Enhanced arsenic removal from aqueous solution by Fe/Mn-C layered double hydroxide composite. Adsorp Sci Technol
Wu WC, Wang SL, Tzou YM, Chen JH, Wang MK (2007) The adsorption and catalytic transformations of chromium on Mn substituted goethite. Appl Catal B 75:272–280
Wu Z, He M, Guo X, Zhou R (2010) Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: competing ion effect and the mechanism analysis. Sep Purif Technol 76(2):184–190
Xiong H, Zhou L (2008) Synthesis of iron oxyhydroxides of different crystal forms and their roles in adsorption and removal of Cr (VI) from aqueous solutions. Acta Petrologica Et Mineralogica 6
Xu W, Wang H, Liu R, Xu Z, Qu J (2011) The mechanism of antimony(III) removal and its reactions on the surfaces of Fe-Mn binary oxide. J Colloid Interface Sci 363:320–326
Yang K, Liu Y, Li Y, Cao Z, Zhou C, Wang Z, Zhou X, Baig SA, Xu X (2019) Applications and characteristics of Fe-Mn binary oxides for Sb(V) removal in textile wastewater: selective adsorption and the fixed-bed column study. Chemosphere 232:254–263
Yang J, Zhou L, Ma F, Zhao H, Li A (2020) Magnetic nanocomposite microbial extracellular polymeric substances@Fe3O4 supported nZVI for Sb(V) reduction and adsorption under aerobic and anaerobic conditions. Environ Res 189:109950
Zhang H, Hu X (2019) Bioadsorption and microbe-mediated reduction of Sb(V) by a marine bacterium in the presence of sulfite/thiosulfate and the mechanism study. Chem Eng J 359:755–764
Zhang C, Jiang H, Deng Y, Wang A (2019) Adsorption performance of antimony by modified iron powder. RSC Adv 9(54):31645–31653
Zhang J, Wang C, Yang B, Zhang B, Zhao X (2014) Removal of antimony contaminants from water by EC. J Environ Eng 8(10):4244–4248 ((In Chinese))
Zhu J, Wu F, Pan X, Guo J, Wen D (2011) Removal of antimony from antimony mine flotation wastewater by electrocoagulation with aluminum electrodes. J Environ Sci 23(7):1066–1071
Zhao Z, Wang X, Zhao C, Zhu X, Du S (2010) Adsorption and desorption of antimony acetate on sodium montmorillonite. J Colloid Interface Sci 345(2):154–159
Funding
This work was supported by the National Key R&D Program of China (2019YFC0408400).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Yongchao Zhou: conceptualization, methodology. Wenxin Zheng: investigation; writing- original draft preparation. Wenming Zhang: supervision, writing- reviewing and editing. Yiping Zhang: methodology, writing- original draft preparation and supervision. Lei Fang: investigation, writing- reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Weiming Zhang
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Zheng, W., Zhang, W. et al. Effective removal of Sb(V) from aqueous solutions by electrocoagulation with composite scrap iron-manganese as an anode. Environ Sci Pollut Res 29, 58088–58096 (2022). https://doi.org/10.1007/s11356-022-20033-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20033-3