Abstract
The feasibility of removal of chemical oxygen demand (COD) and ammonia nitrogen (NH4+–N) from landfill leachate by an electrochemical assisted HClO/Fe2+ process is demonstrated for the first time. The performance of active chlorine generation at the anode was evaluated in Na2SO4/NaCl media, and a higher amount of active chlorine was produced at greater chloride concentration and higher current density. The probe experiments confirmed the coexistence of hydroxyl radical (•OH) and Fe(IV)-oxo complex (FeIVO2+) in the HClO/Fe2+ system. The influence of initial pH, Fe2+ concentration, and applied current density on COD and NH4+–N abatement was elaborately investigated. The optimum pH was found to be 3.0, and the proper increase in Fe2+ dosage and current density resulted in higher COD removal due to the accelerated accumulation of •OH and FeIVO2+ in the bulk liquid phase, whereas, the NH4+–N oxidation was significantly affected by the applied current density because of the effective active chlorine generation at higher current but was nearly independent of Fe2+ concentration. The reaction mechanism of electrochemical assisted HClO/Fe2+ treatment of landfill leachate was finally proposed. The powerful •OH and FeIVO2+, in concomitance with active chlorine and M(•OH), were responsible for COD abatement, and active chlorine played a key role in NH4+–N oxidation. The proposed electrochemical assisted HClO/Fe2+ process is a promising alternative for the treatment of refractory landfill leachate.
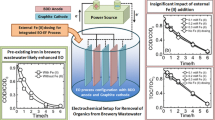
Graphical abstract








Similar content being viewed by others
Data availability
All data and materials used during this study are included in the submitted manuscript and the supplementary files.
References
Abunama T, Othman F, Younes MK (2018) Predicting sanitary landfill leachate generation in humid regions using ANFIS modeling. Environ Monit Assess 190:597
Aguilar ZG, Brillas E, Salazar M, Nava JL, Sirés I (2017) Evidence of Fenton-like reaction with active chlorine during the electrocatalytic oxidation of acid yellow 36 azo dye with Ir-Sn-Sb oxide anode in the presence of iron ion. Appl Catal B Environ 206:44–52
Bunce NJ, Bejan D (2011) Mechanism of electrochemical oxidation of ammonia. Electrochim Acta 56(24):8085–8093
Cabeza A, Urtiaga A, Rivero MJ, Ortiz I (2007) Ammonium removal from landfill leachate by anodic oxidation. J Hazard Mater 144:715–719
Cornejo OM, Sirés I, Nava JL (2020) Electrosynthesis of hydrogen peroxide sustained by anodic oxygen evolution in a flow-through reactor. J Electroanal Chem 873:114419
Costa AM, Alfaia RG de SM, Campos JC (2019) Landfill leachate treatment in Brazil—an overview. J Environ Manag 232: 110-116
Deng Y, Chen N, Hu W, Wang H, Kuang P, Chen F, Feng C (2021) Treatment of old landfill leachate by persulfate enhanced electro-coagulation system: improving organic matters removal and precipitates settling performance. Chem Eng J 424:130262
Deng Y, Zhu X, Chen N, Feng C, Wang H, Kuang P, Hu W (2020) Review on electrochemical system for landfill leachate treatment: performance, mechanism, application, shortcoming, and improvement scheme. Sci Total Environ 745:140768
El Kateb M, Trellu C, Darwich A, Rivallin M, Bechelany M, Nagarajan S, Lacour S, Bellakhal N, Lesage G, Héran M, Cretin M (2019) Electrochemical advanced oxidation processes using novel electrode materials for mineralization and biodegradability enhancement of nanofiltration concentrate of landfill leachates. Water Res 162:446–455
Fang JH, Cai Y, Shen S, Gu L (2022) New insights into FeS/persulfate system for tetracycline elimination: iron valence, homogeneous-heterogeneous reactions and degradation pathways. J Environ Sci 112:48–58
Feng M, Cizmas L, Wang Z, Sharma VK (2017) Activation of ferrate (VI) by ammonia in oxidation of flumequine: kinetics, transformation products, and antibacterial activity assessment. Chem Eng J 323:584–591
Fernandes A, Pacheco MJ, Ciríaco L, Lopes A (2015) Review on the electrochemical processes for the treatment of sanitary landfill leachates: present and future. Appl Catal b: Environ 176–177:183–200
Fu S, Jia H, Meng X, Guo Z, Wang J (2021) Fe-C micro-electrolysis electrocoagulation based on BFDA in the pre-treatment of landfill leachate: enhanced mechanism and electrode decay monitoring. Sci Total Environ 781:146797
Gao Y, Zhou Y, Pang S, Wang Z, Shen Y, Jiang J (2020) Quantitative evaluation of relative contribution of high-valent iron species and sulfate radical in Fe(VI) enhanced oxidation processes via sulfur reducing agents activation. Chem Eng J 387:124077
Garcia-Espinoza JD, Mijaylova-Nacheva P, Aviles-Flores M (2018) Electrochemical carbamazepine degradation: effect of the generated active chlorine, transformation pathways and toxicity. Chemosphere 192:142–151
Ghahrchi M, Rezaee A (2021) Electrocatalytic ozonation process supplemented by EDTA-Fe complex for improving the mature landfill leachate treatment. Chemosphere 263:127858
Keyikoglu R, Karatas O, Rezania H, Kobya M, Vatanpour V, Khataee A (2021) A review on treatment of membrane concentrates generated from landfill leachate treatment processes. Sep Purif Technol 259:118182
Kishimoto N, Nakamura Y, Kato M, Otsu H (2015) Effect of oxidation–reduction potential on an electrochemical Fenton-type process. Chem Eng J 260:590–595
Kwarciak-Kozłowska A, Fijałkowski KL (2021) Efficiency assessment of municipal landfill leachate treatment during advanced oxidation process (AOP) with biochar adsorption (BC). J Environ Manag 287:112309
Lai L, Zhou H, Zhang H, Ao Z, Pan Z, Chen Q, Xiong Z, Yao G, Lai B (2020) Activation of peroxydisulfate by natural titanomagnetite for atrazine removal via free radicals and high-valent iron-oxo species. Chem Eng J 387:124165
Liang J, Duan X, Xu X, Chen K, Zhang Y, Zhao L, Qiu H, Wang S, Cao X (2021) Persulfate oxidation of sulfamethoxazole by magnetic iron-char composites via nonradical pathways: Fe(IV) versus surface-mediated electron transfer. Environ Sci Technol 55:10077–10086
Liang S, Zhu L, Hua J, Duan W, Yang PT, Wang SL, Wei C, Liu C, Feng C (2020) Fe2+/HClO reaction produces FeIVO2+: an enhanced advanced oxidation process. Environ Sci Technol 54:6406–6414
Mandal P, Yadav MK, Gupta AK, Dubey BK (2020) Chlorine mediated indirect electro-oxidation of ammonia using non-active PbO2 anode: influencing parameters and mechanism identification. Sep Purif Technol 247:116910
Moreira FC, Boaventura RAR, Brillas E, Vilar VJP (2017) Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B Environ 202:217–261
Murrieta MF, Brillas E, Nava JL, Sires I (2020) Photo-assisted electrochemical production of HClO and Fe2+ as Fenton-like reagents in chloride media for sulfamethoxazole degradation. Sep Purif Technol 250:117236
Panizza M, Delucchi M, Sirés I (2010) Electrochemical process for the treatment of landfill leachate. J Appl Electrochem 40(10):1721–1727
Reshadi MAM, Bazargan A, McKay G (2020) A review of the application of adsorbents for landfill leachate treatment: focus on magnetic adsorption. Sci Total Environ 731:138863
Shah AD, Liu Z, Salhi E, Höfer T, Gunten U (2015) Peracetic acid oxidation of saline waters in the absence and presence of H2O2: secondary oxidant and disinfection byproduct formation. Environ Sci Technol 49:1698–1705
Shao B, Dong H, Sun B, Guan X (2018) Role of ferrate (IV) and ferrate (V) in activating ferrate (VI) by calcium sulfite for enhanced oxidation of organic contaminants. Environ Sci Technol 53:894–902
Silva AC, Dezotti M, Sant’Anna JrGL, (2004) Treatment and detoxification of a sanitary landfill leachate. Chemosphere 55(2):207–214
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: today and tomorrow. A Review Environ Sci Pollut Res 21:8336–8367
Tai C, Peng JF, Liu JF, Jiang GB, Zou H (2004) Determination of hydroxyl radicals in advanced oxidation processes with dimethyl sulfoxide trapping and liquid chromatography. Anal Chim Acta 527:73–80
Vanlangendonck Y, Corbisier D, Van Lierde A (2005) Influence of operating conditions on the electro-oxidation rate in wastewaters from power plants (ELONITA™ technique). Water Res 39:3028–3034
Wang Z, Jiang J, Pang S, Zhou Y, Guan C, Gao Y, Li J, Yang Y, Qiu W, Jiang C (2018) Is sulfate radical really generated from peroxydisulfate activated by Iron(II) for environmental decontamination? Environ Sci Technol 52:11276–11284
Wu C, Liu J, Liu S, Li W, Yan L, Shu M, Zhao P, Zhou P, Cao W (2018) Assessment of the health risks and odor concentration of volatile compounds from a municipal solid waste landfill in China. Chemosphere 202:1–8
Yang Y, Liu Z, Demeestere K, Van Hulle S (2021) Ozonation in view of micropollutant removal from biologically treated landfill leachate: removal efficiency •OH exposure, and surrogate-based monitoring. Chem Eng J 410:128413
Ye Z, Brillas E, Centellas F, Cabot PL, Sirés I (2020) Expanding the application of photoelectro-Fenton treatment to urban wastewater using the Fe(III)-EDDS complex. Water Res 169:115219
Ye Z, Zhang H, Zhang X, Zhou D (2016) Treatment of landfill leachate using electrochemically assisted UV/chlorine process: effect of operating conditions, molecular weight distribution and fluorescence EEM-PARAFAC analysis. Chem Eng J 286:508–516
Zhang C, He D, Ma J, Waite TD (2018) Active chlorine mediated ammonia oxidation revisited: reaction mechanism, kinetic modelling and implications. Water Res 145:220–230
Zhang J, Yang P, Zheng J, Li J, Jin S, Lv T, Zou YN, Xu P, Cheng C, Zhang Y (2020) Degradation of gaseous HCHO in a rotating photocatalytic fuel cell system with an absorption efficiency of up to 94%. Chem Eng J 392:123634
Zong Y, Shao Y, Zeng Y, Shao B, Xu L, Zhao Z, Liu W, Wu D (2021) Enhanced oxidation of organic contaminants by iron(II)-activated periodate: the significance of high-valent iron–oxo species. Environ Sci Technol 55:7634–7642
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, China (No. 02190052020062) and the National Natural Science Foundation of China (No. 52100073).
Author information
Authors and Affiliations
Contributions
ZY: conceptualization, methodology, data collection, formal analysis, writing—original draft, funding acquisition.
FM: methodology, data collection, formal analysis, writing—review and editing. HZ: conceptualization, methodology, validation, resources, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Ethics approval
This study follows all ethical practices during writing.
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo A. Torres-Palma
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, Z., Miao, F. & Zhang, H. Performance investigation of electrochemical assisted HClO/Fe2+ process for the treatment of landfill leachate. Environ Sci Pollut Res 29, 46875–46884 (2022). https://doi.org/10.1007/s11356-022-19174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19174-2