Abstract
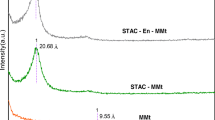
Herein, a new adsorbent was prepared by modifying Mg-Fe LDH for the removal of Cu2+ metal ions from wastewater. Mg-Fe LDH with 5-(3-nitrophenyllazo)-6-aminouracil ligand has been successfully prepared using direct co-precipitation methods and was fully characterized using FTIR analysis, X-ray diffraction, BET surface area theory, zeta potential, partial size, TGA/DTA, CHN, EDX, FESEM, and HRTEM. The surface areas of Mg-Fe LDH and Mg-Fe LDH/ligand were 73.9 m2/g and 34.7 m2/g respectively. Moreover, Cu2+ adsorption on LDH surfaces was intensively examined by adjusting different parameters like time, adsorbent dosage, pH, and Cu2+ metal ion concentration. Several isotherm and kinetic models were investigated to understand the mechanism of adsorption towards Cu2+ metal ions. Adsorption capacity values of LDH and ligand-LDH rounded about 165 and 425 mg/g respectively, applying nonlinear fitting of Freundlich and Langmuir isotherm equations showing that the ligand-LDH can be considered a potential material to produce efficient adsorbent for removal of heavy metal from polluted water. The adsorption of Cu2+ metal ions followed a mixed 1,2-order mechanism. The isoelectric point (PZC) of the prepared sample was investigated and discussed. The effect of coexisting cations on the removal efficiency of Cu2+ ions shows a minor decrease in the adsorption efficiency. Recyclability and chemical stability of these adsorbents show that using Mg-Fe LDH/ligand has an efficiency removal for Cu2+ ions higher than Mg-Fe LDH through seven adsorption/desorption cycles. Moreover, the recycling of the Cu2+ ions was tested using cyclic voltammetry technique from a neutral medium, and the Cu2+ ion recovery was 68%.














Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Ahmed AAA, Talib ZA, Bin Hussein MZ (2012) Thermal, optical and dielectric properties of Zn–Al layered double hydroxide. Appl Clay Sci 56:68–76
Avrami M (1939) Kinetics of phase change. I general theory. J Chem Phys 7(12):1103–1112
Avrami M (1941a) Granulation, phase change, and microstructure kinetics of phase change. III. J Chem Phys 9(2):177–184
Avrami M (1941b) Kinetics of phase change. III: granulation, phase change and microstructure. J Chem Phys 9:177–184
Awual MR (2016) Assessing of lead (III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem Eng J 289:65–73
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. Journal of Chemistry 2017:1–11
Bagheri A, Abu-Danso E, Iqbal J, Bhatnagar A (2020) Modified biochar from Moringa seed powder for the removal of diclofenac from aqueous solution. Environ Sci Pollut Res 27(7):7318–7327
Bakr A, Eshaq G, Rabie A, Mady A, ElMetwally A (2016) Copper ions removal from aqueous solutions by novel Ca–Al–Zn layered double hydroxides. Desalin Water Treat 57(27):12632–12643
Behbahani TJ, Behbahani ZJ (2014) A new study on asphaltene adsorption in porous media. Petroleum & Coal 56(5)
Behbahani, E. S., K. Dashtian and M. Ghaedi (2020). "Fe3O4-FeMoS4: promise magnetite LDH-based adsorbent for simultaneous removal of Pb (II), Cd (II), and Cu (II) heavy metal ions." Journal of hazardous materials: 124560
Chen W, Rakhi R, Alshareef HN (2013) Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J Mater Chem A 1(10):3315–3324
Chen H, Lin J, Zhang N, Chen L, Zhong S, Wang Y, Zhang W, Ling Q (2018) Preparation of MgAl-EDTA-LDH based electrospun nanofiber membrane and its adsorption properties of copper (II) from wastewater. J Hazard Mater 345:1–9
Chimene D, Alge DL, Gaharwar AK (2015) Two-dimensional nanomaterials for biomedical applications: emerging trends and future prospects. Adv Mater 27(45):7261–7284
Choong CE, Wong KT, Jang SB, Saravanan P, Park C, Kim S-H, Jeon B-H, Choi J, Yoon Y, Jang M (2020) Granular Mg-Fe layered double hydroxide prepared using dual polymers: insights into synergistic removal of As (III) and As (V). J Hazard Mater 403:123883
Das N, Konar J, Mohanta M, Srivastava S (2004) Adsorption of Cr (VI) and Se (IV) from their aqueous solutions onto Zr4+−substituted ZnAl/MgAl-layered double hydroxides: effect of Zr4+ substitution in the layer. J Colloid Interface Sci 270(1):1–8
Elmoubarki R, Mahjoubi FZ, Elhalil A, Tounsadi H, Abdennouri M, Sadiq MH, Qourzal S, Zouhri A, Barka N (2017) Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: preparation, characterization and application on textile dyes removal. Journal of Materials Research and Technology 6(3):271–283
El-Reesh GYA, Farghali AA, Taha M, Mahmoud RK (2020) Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr (VI) removal. Sci Rep 10(1):1–20
Farghali A, Tawab HA, Moaty SA, Khaled R (2017) Functionalization of acidified multi-walled carbon nanotubes for removal of heavy metals in aqueous solutions. Journal of Nanostructure in Chemistry 7(2):101–111
Forano C, Costantino U, Prévot V, Gueho CT (2013) Layered double hydroxides (LDH). Developments in clay science, Elsevier 5:745–782
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Gaffer A, Al Kahlawy AA, Aman D (2017) Magnetic zeolite-natural polymer composite for adsorption of chromium (VI). Egypt J Pet 26(4):995–999
González M, Pavlovic I, Barriga C (2015) Cu (II), Pb (II) and Cd (II) sorption on different layered double hydroxides. A kinetic and thermodynamic study and competing factors. Chem Eng J 269:221–228
Gürses A, Doğar Ç, Yalçın M, Açıkyıldız M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater 131(1–3):217–228
Hadi M, McKay G, Samarghandi MR, Maleki A, Solaimany Aminabad M (2012) Prediction of optimum adsorption isotherm: comparison of Chi-square and log-likelihood statistics. Desalin Water Treat 49(1–3):81–94
Hernandez-Moreno MJ, Ulibarri MA, Rendon J, Serna CJ (1985) IR characteristics of hydrotalcite-like compounds. Phys Chem Miner 12(1):34–38
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Ho Y, Ng J, McKay G (2000) Kinetics of pollutant sorption by biosorbents. Separation and Purification Methods 29(2):189–232
Huang G, Jiang L, Wang D, Chen J, Li Z, Ma S (2016) Intercalation of thiacalix [4] arene anion via calcined/restored reaction into LDH and efficient heavy metal capture. J Mol Liq 220:346–353
Hui B, Zhang Y, Ye L (2015) Structure of PVA/gelatin hydrogel beads and adsorption mechanism for advanced Pb (II) removal. J Ind Eng Chem 21:868–876
Imran M, Khan ZUH, Iqbal J, Shah NS, Muzammil S, Ali S, Muhammad N, Aziz A, Murtaza B, Naeem MA (2020a) Potential of siltstone and its composites with biochar and magnetite nanoparticles for the removal of cadmium from contaminated aqueous solutions: batch and column scale studies. Environ Pollut 259:113938
Imran M, Khan ZUH, Iqbal MM, Iqbal J, Shah NS, Munawar S, Ali S, Murtaza B, Naeem MA, Rizwan M (2020b) Effect of biochar modified with magnetite nanoparticles and HNO3 for efficient removal of Cr (VI) from contaminated water: a batch and column scale study. Environ Pollut 261:114231
Iqbal J, Shah NS, Sayed M, Imran M, Muhammad N, Howari FM, Alkhoori SA, Khan JA, Khan ZUH, Bhatnagar A (2019) Synergistic effects of activated carbon and nano-zerovalent copper on the performance of hydroxyapatite-alginate beads for the removal of As3+ from aqueous solution. J Clean Prod 235:875–886
Iwasaki T, Yoshii H, Nakamura H, Watano S (2012) Simple and rapid synthesis of Ni–Fe layered double hydroxide by a new mechanochemical method. Appl Clay Sci 58:120–124
Jaśkaniec S, Hobbs C, Seral-Ascaso A, Coelho J, Browne MP, Tyndall D, Sasaki T, Nicolosi V (2018) Low-temperature synthesis and investigation into the formation mechanism of high quality Ni-Fe layered double hydroxides hexagonal platelets. Sci Rep 8(1):1–8
Jenne EA (1998) Adsorption of metals by geomedia: data analysis, modeling, controlling factors, and related issues. Academic Press, San Diego
Keochaiyom B, Wan J, Zeng G, Huang D, Xue W, Hu L, Huang C, Zhang C, Cheng M (2017) Synthesis and application of magnetic chlorapatite nanoparticles for zinc (II), cadmium (II) and lead (II) removal from water solutions. J Colloid Interface Sci 505:824–835
Koble RA, Corrigan TE (1952) Adsorption isotherms for pure hydrocarbons. Industrial & Engineering Chemistry 44(2):383–387
Kumar Allada R, Navrotsky A, Berbeco HT, Casey WH (2002) Thermochemistry and aqueous solubilities of hydrotalcite-like solids. Science 296(5568):721–723
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Li L, Iqbal J, Zhu Y, Zhang P, Chen W, Bhatnagar A, Du Y (2018) Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Int J Biol Macromol 120:1752–1759
Lingamdinne LP, Chang Y-Y, Yang J-K, Singh J, Choi E-H, Shiratani M, Koduru JR, Attri P (2017) Biogenic reductive preparation of magnetic inverse spinel iron oxide nanoparticles for the adsorption removal of heavy metals. Chem Eng J 307:74–84
Lv L, Chen N, Feng C, Gao Y, Li M (2017) Xanthate-modified magnetic chitosan/poly (vinyl alcohol) adsorbent: preparation, characterization, and performance of Pb (II) removal from aqueous solution. J Taiwan Inst Chem Eng 78:485–492
Mahmoud R, Moaty SA, Mohamed F, Farghali A (2017) Comparative study of single and multiple pollutants system using Ti–Fe chitosan LDH adsorbent with high performance in wastewater treatment. J Chem Eng Data 62(11):3703–3722
Mahmoud RK, Kotp AA, El-Deen AG, Farghali AA, El-Ela FIA (2020) Novel and effective Zn-Al-GA LDH anchored on nanofibers for high-performance heavy metal removal and organic decontamination: bioremediation approach. Water Air Soil Pollut 231(7):1–18
Malash GF, El-Khaiary MI (2010) Methylene blue adsorption by the waste of Abu-Tartour phosphate rock. J Colloid Interface Sci 348(2):537–545
Marczewski A (2010) Application of mixed order rate equations to adsorption of methylene blue on mesoporous carbons. Appl Surf Sci 256(17):5145–5152
Moaty SA, Farghali A, Khaled R (2016) Preparation, characterization and antimicrobial applications of Zn-Fe LDH against MRSA. Mater Sci Eng C 68:184–193
Moaty SA, Farghali A, Moussa M, Khaled R (2017) Remediation of waste water by Co–Fe layered double hydroxide and its catalytic activity. J Taiwan Inst Chem Eng 71:441–453
Moaty SA, Mahmoud RK, Mohamed NA, Gaber Y, Farghali AA, Wahed MS, Younes HA (2018) Synthesis and characterization of LDH-type anionic nanomaterials for effective removal of doxycycline from aqueous media. Microporous Mesoporous Mater 260(2018):84–92
Mohapatra, M. and S. Anand (2010). "Synthesis and applications of nano-structured iron oxides/hydroxides–a review." International Journal of Engineering, Science and Technology 2(8)
Musella E, Gualandi I, Scavetta E, Rivalta A, Venuti E, Christian M, Morandi V, Mullaliu A, Giorgetti M, Tonelli D (2019) Newly developed electrochemical synthesis of Co-based layered double hydroxides: toward noble metal-free electro-catalysis. J Mater Chem A 7(18):11241–11249
Nagendran S, Kamath PV (2017) Synthon approach to structure models for the bayerite-derived layered double hydroxides of Li and Al. Inorg Chem 56(9):5026–5033
Padmavathy K, Madhu G, Haseena P (2016) A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. Procedia Technology 24:585–594
Pérez-Marín A, Zapata VM, Ortuno J, Aguilar M, Sáez J, Lloréns M (2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard Mater 139(1):122–131
Podder M, Majumder C (2016) Studies on the removal of As (III) and As (V) through their adsorption onto granular activated carbon/MnFe2O4 composite: isotherm studies and error analysis. Composite Interfaces 23(4):327–372
Pourfaraj R, Fatemi SJ, Kazemi SY, Biparva P (2017) Synthesis of hexagonal mesoporous MgAl LDH nanoplatelets adsorbent for the effective adsorption of brilliant yellow. J Colloid Interface Sci 508:65–74
Qiu H, Yan J, Lan G, Liu Y, Song X, Peng W, Cui Y (2016) Removal of Cu 2+ from wastewater by modified xanthan gum (XG) with ethylenediamine (EDA). RSC Adv 6(86):83226–83233
Rudzinski W, Plazinski W (2008) Kinetics of dyes adsorption at the solid− solution interfaces: a theoretical description based on the two-step kinetic model. Environmental Science & Technology 42(7):2470–2475
Senthilkumaar S, Varadarajan P, Porkodi K, Subbhuraam C (2005) Adsorption of methylene blue onto jute fiber carbon: kinetics and equilibrium studies. J Colloid Interface Sci 284(1):78–82
Sepehr MN, Al-Musawi TJ, Ghahramani E, Kazemian H, Zarrabi M (2017) Adsorption performance of magnesium/aluminum layered double hydroxide nanoparticles for metronidazole from aqueous solution. Arab J Chem 10(5):611–623
Shahat A, Awual MR, Khaleque MA, Alam MZ, Naushad M, Chowdhury AS (2015) Large-pore diameter nano-adsorbent and its application for rapid lead (II) detection and removal from aqueous media. Chem Eng J 273:286–295
Sokol D, Vieira DE, Zarkov A, Ferreira MG, Beganskiene A, Rubanik VV, Shilin AD, Kareiva A, Salak AN (2019) Sonication accelerated formation of Mg-Al-phosphate layered double hydroxide via sol-gel prepared mixed metal oxides. Sci Rep 9(1):1–9
Son E-B, Poo K-M, Mohamed HO, Choi Y-J, Cho W-C, Chae K-J (2018) A novel approach to developing a reusable marine macro-algae adsorbent with chitosan and ferric oxide for simultaneous efficient heavy metal removal and easy magnetic separation. Bioresour Technol 259:381–387
Srivastava S, Agrawal S, Mondal M (2015) Biosorption isotherms and kinetics on removal of Cr (VI) using native and chemically modified Lagerstroemia speciosa bark. Ecol Eng 85:56–66
Vuković GD, Marinković AD, Škapin SD, Ristić MĐ, Aleksić R, Perić-Grujić AA, Uskoković PS (2011) Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem Eng J 173(3):855–865
Wanees SA, Ahmed AMM, Adam MS, Mohamed MA (2012) Adsorption studies on the removal of hexavalent chromium-contaminated wastewater using activated carbon and bentonite. Chem J 2(3):95–105
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. AICHE J 20(2):228–238
Xie Y, Yuan X, Wu Z, Zeng G, Jiang L, Peng X, Li H (2019) Adsorption behavior and mechanism of Mg/Fe layered double hydroxide with Fe3O4-carbon spheres on the removal of Pb (II) and Cu (II). J Colloid Interface Sci 536:440–455
Yang QZ, Yang J, Zhang CK (2006) Synthesis and properties of cordycepin intercalates of Mg–Al–nitrate layered double hydroxides. Int J Pharm 326(1–2):148–152
Yang W, Zhou M, Oturan N, Bechelany M, Cretin M, Oturan MA (2020) Highly efficient and stable FeIIFeIII LDH carbon felt cathode for removal of pharmaceutical ofloxacin at neutral pH. J Hazard Mater 122513
Younes HA, Khaled R, Mahmoud HM, Nassar HF, Abdelrahman MM, El-Ela FIA, Taha M (2019) Computational and experimental studies on the efficient removal of diclofenac from water using ZnFe-layered double hydroxide as an environmentally benign absorbent. J Taiwan Inst Chem Eng 102:297–311
Zaher A, Taha M, Farghali AA, Mahmoud RK (2020) Zn/Fe LDH as a clay-like adsorbent for the removal of oxytetracycline from water: combining experimental results and molecular simulations to understand the removal mechanism. Environ Sci Pollut Res:1–14
Zaki, Z., S. Abbas, H. Dessoukii, H. Awes and R. Mahmoud (2018). Synthesis, structural elucidation and antimicrobial activities of 5-(3-nitrophenyllazo)-6-aminouracil and its complexes with some transition metal ions. IOP Conference Series: Materials Science and Engineering, IOP Publishing
Zawrah M, Ghanaym EE, Sadek H, El Defrawy S, Ali OA (2019) Synthesis, characterization and sinterability of pure and Ni-doped nano layered double hydroxides from aluminum dross. Ceram Int 45(14):17598–17610
Zhou H, Tan Y, Gao W, Zhang Y, Yang Y (2020) Removal of copper ions from aqueous solution by a hydrotalcite-like absorbent FeMnMg-LDH. Water Air Soil Pollut 231(7):1–12
Zubair M, Daud M, McKay G, Shehzad F, Al-Harthi MA (2017) Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl Clay Sci 143:279–292
Author information
Authors and Affiliations
Contributions
Dr. Rehab K. Mahmoud and Zinat Zaki conceived the idea for water treatment application; participated in study design, interpretation, and data analysis of treatment application; and wrote and revised the manuscript by Prof. Dr. Hassan Dessoukii. Hanna Awes and Safa Abbas prepared the mentioned nanomaterials. S.A. Abd El Moaty and Amal Zaher wrote aforementioned characterizations of the prepared nanomaterials. Amal Zaher, Nabila Shehata, and Ahmed Farghali performed the water remediation from cupper metal ion studies.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Angeles Blanco
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 428 kb)
Rights and permissions
About this article
Cite this article
Awes, H., Zaki, Z., Abbas, S. et al. Removal of Cu2+ metal ions from water using Mg-Fe layered double hydroxide and Mg-Fe LDH/5-(3-nitrophenyllazo)-6-aminouracil nanocomposite for enhancing adsorption properties. Environ Sci Pollut Res 28, 47651–47667 (2021). https://doi.org/10.1007/s11356-021-13685-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13685-0