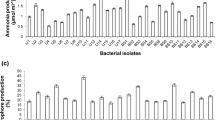
Abstract
The strain SCPG-7 was isolated from saline soil in a cotton field. It is confirmed that the strain SCPG-7 is Pseudomonas sp. by means of the analysis of its phenotypic features and 16S rRNA sequence. SCPG-7 was capable of dissolving mineral tri-calcium phosphate (Ca3(PO4)2) and tri-magnesium phosphate (Mg3(PO4)2). In contrast, no showing iron phosphate (FePO4) or aluminum phosphate (AlPO4) solubilizing activities were detected by this experimental approach. The ratio of the dissolved P diameter to the colony diameter was 1.86. To study the phosphate dissolving mechanisms of the strain, we analyzed the changes of the pH value, the soluble phosphate content, the concentration of alkaline phosphatase, and the production of organic acid in the insoluble phosphate liquid medium. 2-keto-D-gluconicacid, α-ketoglutaric acid, succinic acid, etc. were characterized by LC-MS/MS in NBRIP medium. The concentration of 2-keto-D-gluconicacid increased to 88.6 mg/L after being cultured for 216 h. The strain decreased the pH value of the medium from 7.4 to 4.7 and the released soluble phosphate up to 516 mg/L, which proved the production of organic acids and alkaline phosphatase to be mechanism for solubilizing P. Under low phosphorus stress, Pseudomonas global regulatory protein PhoB regulates the transcription of the alkaline phosphatase gene. IAA and siderophore were secreted by SCPG-7. After treatment with SCPG-7, the individual plant height and dry weight of pepper increased by 23.3 and 31.2%, respectively, compared to the control group. The results show that the strain SCPG-7 has the potential to convert insoluble inorganic phosphorus to plant-available phosphorus. It can enhance soil phosphorus release through biological pathways, thereby increasing crop yield, and providing germplasm resources for the development of biological fertilizers.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Alsohim AS, Taylor TB, Barrett GA, Gallie J, Jackson RW (2014) The biosurfactant viscosin produced by Pseudomonas fluorescens SBW25 aids spreading motility and plant growth promotion. Environ Microbiol 16:2267–2281
Arnaud J, Nausicaa N, Alexandre V, Baekel A, LiAmand G (2019) Influence of edaphic conditions and nitrogen fertilizers on cadmium and zinc phytoextraction efficiency of Noccaea caerulescens. Sci Total Environ 15:645–659
Brenner (2005) In: Don J, Krieg, Noel R, Staley, James R (ed) Bergey’s manual of systematic bacteriology, 2rd edn. Springer, East Lansing, pp 323–443
Browne P, Rice O, Miller SH, Burke J, Dowling DN, Morrissey JP, O’Gara F (2009) Superior inorganic phosphate solubilization is linked to phylogeny within the Pseudomonas fluorescens complex. Appl Soil Ecol 43:131–138
Chabot R, Antoun H, Cescas MP (1996) Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli. Plant Soil 184:311–321
Cuartero J, Fernández-Muñoz R (1998) Tomato and salinity. Sci Hortic 78:83–125
Dowd C (2004) Gene expression profile changes in cotton root and hypocotyl tissues in response to infection with Fusarium oxysporum f. sp. vasinfectum. Mol Plant Microbe Interact 17:654–667
Etesami H, Hosseini HM, Alikhani HA, Mohammadi L (2014) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul 33:654–670
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Goldstein AH (2009) Bacterial solubilization of mineral phosphates: historical perspective and future prospects. Am J Altern Agric 1:51–57
Gon.Alves MR, Couto AA, Maria FE, Breda BDH, Ventura MGM, Honor MA (2009) Root colonization and interaction among growth promoting rhizobacteria isolates and eucalypts species. Rev Árvore 33:1–9
Irshad U, Villenave C, Brauman A, Plassard C (2011) Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol Biochem 43:2121–2126
Kasim K, Pallavi A, Arti S, Aniruddha SV, Manoj P (2015) Heterologous expression of two Jatropha Aquaporins imparts drought and salt tolerance and improves seed viability in transgenic Arabidopsis thaliana. PLoS One 10:1–20
Khanna K, Jamwal VL, Sharma A, Gandhi SG, Ohri P, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ali HM, Ahmad P (2019) Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 230:628–639
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
Krishna R, Karkute SG, Ansari WA, Jaiswal DK, Verma JP, Singh M (2019) Transgenic tomatoes for abiotic stress tolerance: status and way ahead. 3 Biotech 9:1–14
Kumar V, Singh S, Singh R, Upadhyay N, Singh J (2017) Design, synthesis, and characterization of 2,2-bis(2,4-dinitrophenyl)-2-(phosphonatomethylamino)acetate as a herbicidal and biological active agent. J Chem Biol 10:179–190
Lidbury IDEA, Murphy ARJ, Scanlan DJ, Bending GD, Jones AME, Moore JD, Goodall A, Hammond JP, Wellington EMH (2016) Comparative genomic, proteomic and exoproteomic analyses of three Pseudomonas strains reveals novel insights into the phosphorus scavenging capabilities of soil bacteria. Environ Microbiol 18:3535–3549
Long XE, Yao H, Huang Y, Wei W, Zhu Y-G (2018) Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol Biochem 118:103–114
Mander C, Wakelin S, Young S, Condron L, Oon L, Okelin S, Young S, Condron L, O’Callaghan M (2012) Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol Biochem 44:93–101
Martinez V, Läuchli A (2006) Salt-induced inhibition of phosphate uptake in plants of cotton (Gossypium hirsutum L.). New Phytol 126:609–614
Mirza MS, Mehnaz S, Normand P, Prigent-Combaret C, Monne-Loccoz Y, Bally R, Malik KA (2006) Molecular characterization and PCR detection of a nitrogen-fixing Pseudomonasstrain promoting rice growth. Biol Fertil Soils 43:163–170
Monds RD, Newell PD, Schwartzman JA (2006) Conservation of the Pho regulon in Pseudomonas fluorescens Pf0-1. Appl Environ Microbiol 72:1910–1924
Nicholas O, Lally RD, Samuel K, Andrew L, David R, Germaine KJ, Dowling DN (2015) Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol 6:1–9
O’Brien PJ, Herschlag D (1998) Sulfatase activity of E. coli alkaline phosphatase demonstrates a functional link to Arylsulfatases, an evolutionarily related enzyme family. J Am Chem Soc 120:12369–12370
Oberhansli T, Defago G, Haas D (1991) Indole-3-acetic acid (IAA) synthesis in the biocontrol strain CHA0 of Pseudomonas fluorescens: role of tryptophan side chain oxidase. J Gen Microbiol 137:2273–2279
Pérez E, Sulbarán M, Ball MM, Yarzábal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39:2905–2914
Ribaut JM, Pilet PE (1994) Water stress and indol-3yl-acetic acid content of maize roots. Planta 193:502–507
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Sturz AV, Matheson BG, Arsenault W, Kimpinski J, Christie BR (2001) Weeds as a source of plant growth promoting rhizobacteria in agricultural soils. Can J Microbiol 47:1013–1024
Sun WJ, Yu L, Yu SL, Cui FJ, Zhou YZ, Zhou Q, Sun L (2012) Kinetic modeling of 2-keto-gluconic acid (2KGA) poduction from rice starch hydrolysate ising Pseudomonas fluorescens AR4. Adv Mater Res 550-553:1144–1150
Sun J, Li YP, Suo C, Liu J (2020) Development of an uncertain water-food-energy nexus model for pursuing sustainable agricultural and electric productions. Agric Water Manag 241:106384
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Whitelaw MA, Harden TJ, Helyar KR (1999) Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil Biol Biochem 31:655–665
Yao LL, Ye BC (2015) The reciprocal regulation of GlnR and PhoP in response to nitrogen and phosphate limitations in Saccharopolyspora erythraea. Appl Environ Microbiol 82:807–808
Yu X, Ai C, Xin L, Zhou G (2011) The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur J Soil Biol 47:138–145
Yuan ZC, Rahat Z, Richard M, Finan TM (2006) Genome prediction of PhoB regulated promoters in Sinorhizobium meliloti and twelve proteobacteria. Nucleic Acids Res 34:2686–2697
Funding
This work was supported by the National Natural Science Foundation of China (grant 21575089) and Scientific Research Foundation for Changjiang Scholars of Shihezi University (CJXZ201501)
Author information
Authors and Affiliations
Contributions
Yajie Han conceived the study, designed the study, and collected the data. Shengxue Liu, Fulong Chen, Xiaolin Deng, Zhuang Miao, Zhansheng Wu, and Bang-Ce Ye analyzed the data and were involved in writing the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Yes.
Consent to publish
Yes.
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(TXT 1 kb).
Rights and permissions
About this article
Cite this article
Han, Y., Liu, S., Chen, F. et al. Characteristics of plant growth–promoting rhizobacteria SCPG-7 and its effect on the growth of Capsicum annuum L.. Environ Sci Pollut Res 28, 11323–11332 (2021). https://doi.org/10.1007/s11356-020-11388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11388-6