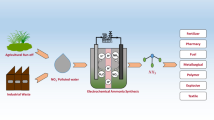
Abstract
Membrane separation processes are being currently applied to produce drinking water from water contaminated with nitrate. The overall process generates a brine with high nitrate/nitrite concentration that is usually send back to a conventional wastewater treatment plant. Catalytic processes to nitrate reduction are being studied, but the main goal of achieving a high selectivity to nitrogen production is still a matter of research. In this work, a two-step process was evaluated, aiming to verify the best combination of operational parameters to efficiently reduce nitrate to nitrogen. In the first step, the nitrate was reduced to nitrite by electroreduction, applying a copper electrode and different cell potentials. A second step of the process was carried out by reducing the generated nitrite with a catalytic process by hydrogenation. The results showed that the highest nitrate reduction (89%) occurred when a cell potential of 11 V was applied. In this condition, the nitrite ion was generated with all experimental conditions evaluated. Then, to reduce the nitrite ion formed by catalytic reduction, activated carbon fibers (ACF) and powder γ-alumina (γ-Al2O3) were tested as supports for palladium (Pd). With both catalysts, the total nitrite conversion was obtained, being the selectivity to gaseous compounds 94% and 97% for Pd/Al2O3 and Pd/ACF, respectively. Considering the results obtained, a two-stage treatment setup to brine denitrification may be proposed. With electrochemistry, an operating condition was achieved in which ammonium production can be controlled to very low values, but the reduction is predominant to nitrite. With the second step, all nitrite is converted to nitrogen gas and just 3% of ammonium is produced with the most selective catalyst. The main novelty of this work is associated to the use of a two-stage process enabling 89% of nitrate reduction and 100% of nitrite reduction.









Similar content being viewed by others
References
Banasiak LJ, Kruttschnitt TW, Schäfer AI (2007) Desalination using electrodialysis as a function of voltage and salt concentration. Desalination 205:38–46. https://doi.org/10.1016/j.desal.2006.04.038
Barrabés N, Sá J (2011) Catalytic nitrate removal from water, past, present and future perspectives. Appl Catal B 104:1–5. https://doi.org/10.1016/j.apcatb.2011.03.011
Beltrame TF, Coelho V, Marder L, Ferreira JZ, Marchesini FA, Bernardes AM (2017) Effect of operational parameters and Pd/In catalyst in the reduction of nitrate using copper electrode. Environ Technol 39:2835–2847. https://doi.org/10.1080/09593330.2017.1367422
Bernardes AM, Dalla Costa RF, Fallavena VLV, Rodrigues MAS, Trevisan MD, Ferreira JZ (2000) Electrochemistry as a clean technology for the treatment of effluents: the application of electrodialysis. Met Finish 98(52–58):114
Bi J, Peng C, Xu H, Ahmed A-S (2011) Removal of nitrate from groundwater using the technology of electrodialysis and electrodeionization. Desalination and Water Treat 34:394–401. https://doi.org/10.5004/dwt.2011.2891
Bittencourt SD, Marder L, Benvenuti T, Ferreira JZ, Bernardes AM (2017) Analysis of different current density conditions in the electrodialysis of zinc electroplating process solution. Sep Sci Technol 52:2079–2089. https://doi.org/10.1080/01496395.2017.1310896
Bosko ML, Marchesini FA, Cornaglia LM, Miró EE (2011) Controlled Pd deposition on carbon fibers by electroless plating for the reduction of nitrite in water. Catal Commun 16:189–193. https://doi.org/10.1016/j.catcom.2011.09.034
Bosko ML, Rodrigues MAS, Ferreira JZ, Miró EE, Bernardes AM (2014) Nitrate reduction of brines from water desalination plants by membrane electrolysis. J MembrSci 451:276–284. https://doi.org/10.1016/j.memsci.2013.10.004
Bouzek K, Paidar M, Sadilkova A, Bergmann H (2001) Electrochemical reduction of nitrate in weakly alkaline solutions. J Appl Electrochem 31:1185–1193
Cheikh A, Grib H, Drouiche N, Abdi N, Lounici H, Mameri N (2013) Water denitrification by a hybrid process combining a new bioreactor and conventional electrodialysis. Chem Eng Process 63:1–6
Cheng H, Scott K, Christensen PA (2005) Application of a solid polymer electrolyte reactor to remove nitrate ions from wastewater. J Appl Electrochem 35:551–560
Chinthaginjala JK, Lefferts L (2010) Support effect on selectivity of nitrite reduction in water. Appl Catal B 101:144–149. https://doi.org/10.1016/j.apcatb.2010.09.023
Choi E, Park K, Lee H, Cho M, Ahn S (2013) Formic acid as an alternative reducing agent for the catalytic nitrate reduction in aqueous media. J Environ Sci 25:1696–1702. https://doi.org/10.1016/S1001-0742(12)60226-5
Cobo M, Quintero A, de Correa CM (2008) Liquid phase dioxin hydrodechlorination over Pd/γ–Al2O3. Catal Today 133–135:509–519. https://doi.org/10.1016/j.cattod.2007.12.020
de Groot M, Koper MT (2004) The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J Electroanal Chem 562:81–94. https://doi.org/10.1016/j.jelechem.2003.08.011
De Lucas A, Rodríguez L, Villaseñor J, Fernández FJ (2005) Denitrification potential of industrial wastewaters. Water Res 39:3715–3726. https://doi.org/10.1016/j.watres.2005.06.024
Deganello F, Liotta L, Macaluso A, Venezia A, Deganello G (2000) Catalytic reduction of nitrates and nitrites in water solution on pumice-supported Pd–Cu catalysts. Appl Catal B 24:265–273. https://doi.org/10.1016/S0926-3373(99)00109-5
Devard A, Ulla MA, Marchesini FA (2013) Synthesis of Pd/Al2O3 coating onto a cordierite monolith and its application to nitrite reduction in water. Catal Commun 34:26–29. https://doi.org/10.1016/j.catcom.2013.01.005
Ding J, Li W, Zhao Q-L, Wang K, Zheng Z, Gao Y-Z (2015) Electroreduction of nitrate in water: role of cathode and cell configuration. Chem Eng J 271:252–259. https://doi.org/10.1016/j.cej.2015.03.001
Gao Z, Zhang Y, Li D, Werth CJ, Zhang Y, Zhou X (2015) Highly active Pd–In/mesoporous alumina catalyst for nitrate reduction. J Hazard Mater 286:425–431. https://doi.org/10.1016/j.jhazmat.2015.01.005
Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, Westerhoff P (2018) Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl Catal B 236:546–568. https://doi.org/10.1016/j.apcatb.2018.05.041
Guo L, Lv T, He K, Wu S, Dong X, Dong R (2017) Removal of organic matter, nitrogen and faecal indicators from diluted anaerobically digested slurry using tidal flow constructed wetlands. Environ Sci Pollut Res 24:5486–5496. https://doi.org/10.1007/s11356-016-8297-2
Hell F, Lahnsteiner J, Frischherz H, Baumgartner G (1998) Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 117:173–180
Jaworski MA, Lick ID, Siri GJ, Casella ML (2014) ZrO2-modified Al2O3-supported PdCu catalysts for the water denitrification reaction. Appl Catal B 156–157:53–61. https://doi.org/10.1016/j.apcatb.2014.02.048
Jaworski MA, Vetere V, Bideberripe HP, Siri GJ, Casella ML (2013) Structural aspects of PtSn/γ-Al2O3 catalysts prepared through surface-controlled reactions: Behavior in the water denitrification reaction. Appl Catal A 453:227–234. https://doi.org/10.1016/j.apcata.2012.12.034
Jung S, Bae S, Lee W (2014) Development of Pd-Cu/Hematite catalyst for selective nitrate reduction. Environ Sci Technol 48:9651–9658
Kapoor A, Viraraghavan T (1997) Nitrate removal from drinking water. J Environ Eng 123:371–380
Katsounaros I, Ipsakis D, Polatides C, Kyriacou G (2006) Efficient electrochemical reduction of nitrate to nitrogen on tin cathode at very high cathodic potentials. Electrochim Acta 52:1329–1338. https://doi.org/10.1016/j.electacta.2006.07.034
Kim J, Kelly MJ, Lamb HH, Roberts GW, Kiserow DJ (2008) Characterization of palladium (Pd) on alumina catalysts prepared using liquid carbon dioxide. The J Phys Chem C 112:10446–10452. https://doi.org/10.1021/jp711495n
Kim YJ, Lee K, Cha HY, Yoo KM, Jeon CS, Kim HJ, Kim D, Park KY (2015) Electrolytic denitrification with an ion-exchange membrane in groundwater. Water Sci Technol 15:1320–1325. https://doi.org/10.2166/ws.2015.079
Kizito S, Lv T, Wua S, Ajmal Z, Luo H, Dong R (2017) Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: Role of media and tidal operation. Sci Total Environ 592:197–205. https://doi.org/10.1016/j.scitotenv.2017.03.125
Lan H, Mao R, Tong Y, Liu Y, Liu H, An X, Liu R (2016) Enhanced electroreductive removal of bromate by a supported Pd–In Bimetallic catalyst: kinetics and mechanism investigation. Environ Sci Technol 50(21):11872–11878. https://doi.org/10.1021/acs.est.6b02822
Liedtke A-K, Bornette F, Philippe R, de Bellefon C (2016) External liquid solid mass transfer for solid particles transported in a milli-channel within a gas–liquid segmented flow. Chem Eng J 287:92–102. https://doi.org/10.1016/j.cej.2015.10.109
Mahmoud MHH, Abdel-Aal EA, Abdel-hamed RM, Kandil AT (2015) Denitration of Coke plant wastewater using a bench-scale electrodialysis unit via statistical design. Int. J Electrochem Sci 10:1478–1493
Marchesini FA, Gutierrez LB, Querini CA, Miró EE (2010) Pt,In and Pd,In catalysts for the hydrogenation of nitrates and nitrites in water. FTIR characterization and reaction studies. Chem Eng J 159:203–211. https://doi.org/10.1016/j.cej.2010.02.056
Marchesini FA, Irusta S, Querini C, Miró E (2008a) Spectroscopic and catalytic characterization of Pd–In and Pt–In supported on Al2O3 and SiO2, active catalysts for nitrate hydrogenation. Appl Catal A 348:60–70. https://doi.org/10.1016/j.apcata.2008.06.026
Marchesini FA, Irusta S, Querini C, Miró E (2008b) Nitrate hydrogenation over Pt,In/Al2O3 and Pt,In/SiO2. Effect of aqueous media and catalyst surface properties upon the catalytic activity. Catal Commun 9:1021–1026. https://doi.org/10.1016/j.catcom.2007.09.037
Martínez J, Ortíz A, Ortíz I (2017) State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl Catal B 207:42–59. https://doi.org/10.1016/j.apcatb.2017.02.016
Matějŭ V, Čižinská S, Krejčí J, Janoch T (1992) Biological water denitrification—a review. Enzyme Microb Tech 14:170–183
Mattarozzi L, Cattarin S, Comisso N, Guerriero P, Musiani M, Vázquez-Gómez L, Verlato E (2013) Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes. Electrochim Acta 89:488–496. https://doi.org/10.1016/j.electacta.2012.11.074
Menkouchi Sahli MA, Annouar S, Mountadar M, Soufiane A, Elmidaoui A (2008) Nitrate removal of brackish underground water by chemical adsorption and by electrodialysis. Desalination 227:327–333. https://doi.org/10.1016/j.desal.2007.07.021
Mirabi M, Ghaderi E, Rasouli Sadabad H (2017) Nitrate reduction using hybrid system consisting of zero valent magnesium powder/activated carbon (Mg 0 /AC) from water. Process Saf Environ Prot 111:627–634. https://doi.org/10.1016/j.psep.2017.08.035
Naumkin AV, Kraut-Vass A, Gaarenstroom SW, Powell CJ (2014) NIST X-ray Photoelectron Spectroscopy Database. National Institute of Standards and Technology
Palin G (1969) Electrochemistry for Technologists. Pergamon Press Oxford, England
Pérez-Gallent E, Figueiredo MC, Katsounaros I, Koper MTM (2017) Electrocatalytic reduction of nitrate on copper single crystals in acidic and alkaline solutions. Electrochim Acta 227:77–84. https://doi.org/10.1016/j.electacta.2016.12.147
Pourbaix M (1963) Atlas D’equilibres Electrochimiques. Gauthier-Villars, Paris
Puckett LJ (1995) Identifying the major sources of nutrient water pollution. Environ Sci Technol 29:408A–414A
Reyter D, Bélanger D, Roué L (2008) Study of the electroreduction of nitrate on copper in alkaline solution. Electrochim Acta 53:5977–5984. https://doi.org/10.1016/j.electacta.2008.03.048
Reyter D, Bélanger D, Roué L (2009) Elaboration of Cu − Pd films by coelectrodeposition: application to nitrate electroreduction. J Phys Chem C 113:290–297. https://doi.org/10.1021/jp805484t
Reyter D, Bélanger D, Roué L (2010) Nitrate removal by a paired electrolysis on copper and Ti/IrO2 coupled electrodes – Influence of the anode/cathode surface area ratio. Water Res 44:1918–1926. https://doi.org/10.1016/j.watres.2009.11.037
Reyter D, Bélanger D, Roué L, (2011) Optimization of the cathode material for nitrate removal by a paired electrolysis process. J Hazard Mater 192:507–513. https://doi.org/10.1016/j.jhazmat.2011.05.054
Schoeman JJ, Steyn A (2003) Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 155:15–26
Shrimali M, Singh KP (2001) New methods of nitrate removal from water. Environ Pollut 112:351–359
Sinha X (2005) The electrochemical reduction of nitrate in low conductivity aqueous solutions [master’s thesis]. San Jose State University
Subba Rao AN, Venkatarangaiah VT (2014) Metal oxide-coated anodes in wastewater treatment. Environ Sci Pollut Res 21:3197–3217. https://doi.org/10.1007/s11356-013-2313-6
Tong S, Liu H, Feng C, Chen N, Zhao Y, Xu B, Zhao J, Zhu M (2019) Stimulation impact of electric currents on heterotrophic denitrifying microbial viability and denitrification performance in high concentration nitrate-contaminated wastewater. J Environ Sci 77:363–371. https://doi.org/10.1016/j.jes.2018.09.014
Treybal RE (1987) Mass- Transfer Operations. McGraw-Hill
Wang L, Yao Y, Zhang Z, Sun L, Lu W, Chen W, Chen H (2014) Activated carbon fibers as an excellent partner of Fenton catalyst for dyes decolorization by combination of adsorption and oxidation. Chem Eng J 251:348–354. https://doi.org/10.1016/j.cej.2014.04.088
Wang Y, Qu J, Liu H, Hu C (2007) Adsorption and reduction of nitrate in water on hydrotalcite-supported Pd-Cu catalyst. Catal Today 126:476–482. https://doi.org/10.1016/j.cattod.2007.06.024
Wei N, Cui H, Wang X, Xie X, Wang M, Zhang L, Tian J (2017) Hierarchical assembly of In2O3 nanoparticles on ZnO hollow nanotubes using carbon fibers as templates: enhanced photocatalytic and gas-sensing properties. J Colloid Interface Sci 498:263–270. https://doi.org/10.1016/j.jcis.2017.03.072
Wisniewski C, Persin F, Cherif T, Sandeaux R, Grasmick A, Gavach C (2002) Use of a membrane bioreactor for denitrification of brine from an electrodialysis process. Desalination 149:331–336
Wisniewski C, Persin F, Cherif T, Sandeaux R, Grasmick A, Gavach C (2001) Denitrification of drinking water by the association of an electrodialysis process and a membrane bioreactor: feasibility and application. Desalination 139:199–205
Yang S, Xiao T, Zhang J, Chen Y, Li L (2015) Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution. Sep Purif Technol 143:19–26. https://doi.org/10.1016/j.seppur.2015.01.022
Yang Q, Yao F, Zhong Y, Chen F, Shu X, Sun J, He L, Wu B, Hou K, Wang D, Li X (2019) Metal–organic framework supported palladium nanoparticles: applications and mechanisms Part. Part Syst Charact 1800557:1–19. https://doi.org/10.1002/ppsc.201800557
Yao F, Yang Q, Zhong Y, Shu X, Chen F, Sun J, Ma Y, Fu Z, Wang D, Li X (2019) Indirect electrochemical reduction of nitrate in water using zero-valent titanium anode: factors, kinetics, and mechanism. Water Res 157:191–200. https://doi.org/10.1016/j.watres.2019.03.078
Ye T, Durkin DP, Hu M, Wang X, Banek NA, Wagner MJ, Shuai D (2016) Enhancement of nitrite reduction kinetics on electrospun Pd-carbon nanomaterial catalysts for water purification. ACS Appl Mater Interfaces 8(28):17739–17744. https://doi.org/10.1021/acsami.6b03635
Yoshinaga Y, Akita T, Mikami I, Okuhara T (2002) Hydrogenation of nitrate in water to nitrogen over Pd–Cu supported on active carbon. J Catal 207:37–45. https://doi.org/10.1006/jcat.2002.3529
Zoppas FM, Marchesini FA, Devard A, Bernardes AM, Miró EE (2016) Controlled deposition of Pd and in on carbon fibers by sequential electroless plating for the catalytic reduction of nitrate in water. Catal Commun 78:59–63. https://doi.org/10.1016/j.catcom.2016.02.012
Zoppas FM, Bernardes AM, Miró EE, Marchesini FA (2018a) Nitrate reduction of brines from water desalination plants employing a low metallic charge Pd In Catalyst and formic acid as reducing agent. Catal Lett 148:2572–2584. https://doi.org/10.1007/s10562-018-2429-x
Zoppas FM, Bernardes AM, Miró E, Marchesini FA (2018b) Improving selectivity to dinitrogen using palladium-indium coated on activated carbon fibers: Preparation, characterization and application in water-phase nitrate reduction using formic acid as an alternative reductant source. J Environ Chem Eng 6:4764–4772. https://doi.org/10.1016/j.jece.2018.07.015
Acknowledgments
The authors wish to acknowledge the financial support received from ANPCyT (PICT) (2014-1379, 2014-2408), UNL, the Project CAPES-MERCOSUR (CAPG-BA), CYTED and the Brazilian financial support from FINEP, FAPERGS, CNPq, and CAPES. Thanks also to Florencia Agustina Sosa for the revision of the English usage.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Bingcai Pan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beltrame, T.F., Zoppas, F.M., Marder, L. et al. Use of a two-step process to denitrification of synthetic brines: electroreduction in a dual-chamber cell and catalytic reduction. Environ Sci Pollut Res 27, 1956–1968 (2020). https://doi.org/10.1007/s11356-019-06763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06763-x