Abstract
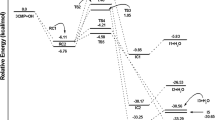
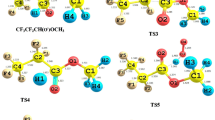
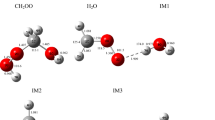
The hydroxyl radical, as the most important oxidant, controls the removal of some volatile organic compounds (VOCs) in the atmosphere. In this work, the atmospheric oxidation processes of acrylic acid by OH radical have been investigated by density functional theory (DFT). The energetic routes of the reaction of CH2CHCOOH with OH radical have been calculated accurately at the CCSD(T)/cc-pVTZ//M06-2X/6-311++G(d,p) level. It is implicated that the oxidation has five elementary reaction pathways mostly hinging on how hydroxyl radical approaches to the carbon skeleton of acrylic acid. The atmospheric degradation mechanisms of the CH2CHCOOH by OH radical are the formation of reactive intermediates IM1 and IM2. Meanwhile, the further oxidation mechanisms of IM1 and IM2 by O3 and NO are also investigated. The rate coefficients have been computed using tight transition state theory of the variflex code. The calculated rate coefficient is 2.3 × 10−11 cm3 molecule−1 s−1 at standard pressure and 298 K, which is very close to the laboratory data (1.75 ± 0.47 × 10−11 cm3 molecule−1 s−1). Moreover, the atmospheric lifetime of acrylic acid is about 6 h at 298 K and 1 atm, implying that the fast sinks of acrylic acid by hydroxyl radical.







Similar content being viewed by others
References
Adamo C, Jacquemin D (2013) The calculations of excited-state properties with time-dependent density functional theory. Chem Soc Rev 42:845–856
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170
Alvarez-Idaboy JR, Mora-Diez N, Vivier-Bunge A (2000) A quantum chemical and classical transition state theory explanation of negative activation energies in OH addition to substituted ethenes. J Am Chem Soc 122:3715–3720
Anglada JM, Solé A (2017) The atmospheric oxidation of HONO by OH, Cl, and ClO radicals. J Phys Chem A 121:9698–9707
Atkinson R (2000) Atmospheric chemistry of VOCs and NOx. Atmos Environ 34:2063–2101
Atkinson R, Arey J (2003) Atmospheric degradation of volatile organic compounds. Chem Rev 103:4605–4638
Baasandorj M, Knight G, Papadimitriou VC, Talukdar RK, Ravishankara AR, Burkholder JB (2010) Rate coefficients for the gas-phase reaction of the hydroxyl radical with CH2=CHF and CH2=CF2. J Phys Chem A 114:4619–4633
Baruah SD, Gour NK, Sarma PJ, Deka RC (2018) OH-initiated mechanistic pathways and kinetics of camphene and fate of product radical: a DFT approach. Environ Sci Pollut Res 25:2147–2156
Becke AD (1993) A new mixing of Hartree–Fock and local density-functional theories. J Chem Phys 98:1372–1377
Berndt T, Scholz W, Mentler B, Fischer L, Herrmann H, Kulmala M, Hansel A (2018) Accretion product formation from self- and cross-reactions of RO2 radicals in the atmosphere. Angew Chem Int Ed Engl 57:3820–3824
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2009) Temperature-dependent rate coefficients for the reactions of Cl atoms with methyl methacrylate, methyl acrylate and butyl methacrylate at atmospheric pressure. Atmos Environ 43:5996–6002
Chapleski RC Jr, Zhang YF, Troya D, Morris JR (2016) Heterogeneous chemistry and reaction dynamics of the atmospheric oxidants, O3, NO3, and OH, on organic surfaces. Chem Soc Rev 45:3731–3746
Cheng L, Yu XJ, Zhao K, Hou H, Wang BS (2017) Electronic structures and OH-induced atmospheric degradation of CF3NSF2: a potential green dielectric replacement for SF6. J Phys Chem A 121:2610–2619
Fang WH, Liu RZ (2000) Photodissociation of acrylic acid in the gas phase: an ab initio study. J Am Chem Soc 122:10886–10894
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, revision D. 01. Gaussian, Inc., Wallingford CT
Gai YB, Ge MF, Wang WG (2011) Kinetics of the gas-phase reactions of some unsaturated alcohols with Cl atoms and O3. Atmos Environ 45:53–59
Galano A, Muñoz-Rugeles L, Alvarez-Idaboy JR, Bao JL, Truhlar DG (2016) Hydrogen abstraction reactions from phenolic compounds by peroxyl radicals: multireference character and density functional theory rate constants. J Phys Chem A 120:4634–4642
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Hein R, Crutzen PJ, Heimann M (1997) An inverse modeling approach to investigate the global atmospheric methane cycle. Glob Biogeochem Cycles 11:43–76
Hippler H, Viskolcz B (2000) Addition complex formation vs. direct abstraction in the OH+ C2H4 reaction. Phys Chem Chem Phys 2:3591–3596
Hratchian HP, Schlegel HB (2005) Using hessian updating to increase the efficiency of a hessian based predictor-corrector reaction path following method. J Chem Theory Comput 1:61–69
Johnston HS, Heicklen J (1962) Tunnelling corrections for unsymmetrical Eckart potential energy barriers. J Phys Chem 66:532–533
Kamal MS, Razzak SA, Hossain MM (2016) Catalytic oxidation of volatile organic compounds (VOCs)—a review. Atmos Environ 140:117–134
Kendall RA, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
Khaled F, Giri BR, Szőri M, Mai TV-T, Huynh LK, Farooq A (2017) A combined high-temperature experimental and theoretical kinetic study of the reaction of dimethyl carbonate with OH radicals. Phys Chem Chem Phys 19:7147–7157
Klippenstein S, Wagner A, Dunbar R, Wardlaw D, Robertson S (1999) VARIFLEX, version 1.00
Kozuch S, Martin Jan ML (2011) The rate-determining step is dead. Long live the rate-determining state! Chem Phys Chem 12:1413–1418
Krapf M, Haddad IE, Bruns EA, Molteni U, Daellenbach KR, Prévôt ASH, Baltensperger U, Dommen J (2016) Labile peroxides in secondary organic aerosol. Cell Chem 1:603–616
Kurylo MJ, Orkin VL (2003) Determination of atmospheric lifetimes via the measurement of OH radical kinetics. Chem Rev 103:5049–5076
Lee TJ, Taylor PR (1989) A diagnostic for determining the quality of single-reference electron correlation methods. Int J Quantum Chem 36:199–207
Lily M, Baidya B, Chandra AK (2017) Theoretical studies on atmospheric chemistry of HFE-245mc and perfluoro-ethyl formate: reaction with OH radicals, atmospheric fate of alkoxy radical and global warming potential. Chem Phys Lett 669:211–217
Lynch BJ, Zhao Y, Truhlar DG (2005) The 6-31B(d) basis set and the BMC-QCISD and BMC-CCSD multicoefficient correlation methods. J Phys Chem A 109:1643–1649
McDonald BC, de Gouw JA, Gilman JB (2018) Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 359:760–764
McLean A, Chandler G (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-18. J Chem Phys 72:5639–5648
Miller JA, Klippenstein SJ, Raffy C (2002) Solution of some one-and two-dimensional master equation models for thermal dissociation: the dissociation of methane in the low-pressure limit. J Phys Chem A 106:4904–4913
Miller JA, Klippenstein SJ (2006) Master equation methods in gas phase chemical kinetics. J Phys Chem A 110:10528–10544
Milhøj BO, Sauer SP (2015) Kinetics and thermodynamics of the reaction between the •OH radical and adenine: a theoretical investigation. J Phys Chem A 119:6516–6527
Murdoch JR (1981) What is the rate-limiting step of a multistep reaction? J Chem Educ 58:32–36
Peiró-García J, Nebot-Gil I (2003) Ab initio study on the mechanism of the atmospheric reaction OH+O3→HO2+O2. Chem Phys Chem 4:843–847
Poling BE, Prausnitz JM, O’Connell JP (2001) The properties of gases and liquids, 5th edn. McGraw-Hill, New York
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:5968–5975
Riva M (2016) Multiphase chemistry of highly oxidized molecules: the case of organic hydroperoxides. Cell Chem 1:522–530
Senosiain JP, Klippenstein SJ, Miller JA (2006) Reaction of ethylene with hydroxyl radicals: a theoretical study. J Phys Chem A 110:6960–6970
Shen XL, Zhao Y, Chen ZM, Huang D (2013) Heterogeneous reactions of volatile organic compounds in the atmosphere. Atmos Environ 68:297–314
Song XL, Zügner GL, Farkas M, Illés Á, Sarzyński D, Rozgonyi T, Wang BS, Dóbé S (2015) Experimental and theoretical study on the OH-reaction kinetics and photochemistry of acetyl fluoride (CH3C (O) F), an atmospheric degradation intermediate of HFC-161 (C2H5F). J Phys Chem A 119:7753–7765
Sun JY, Wang RS, Wang BS (2011) Theoretical study on the gas phase reaction of acrylonitrile with a hydroxyl radical. Phys Chem Chem Phys 13:16585–16595
Teruel MA, Blanco MB, Luque GR (2007) Atmospheric fate of acrylic acid and acrylonitrile: rate constants with Cl atoms and OH radicals in the gas phase. Atmos Environ 41:5769–5777
Vereecken L, Glowacki DR, Pilling MJ (2015) Theoretical chemical kinetics in tropospheric chemistry: methodologies and applications. Chem Rev 115:4063–4114
Yu XJ, Hou H, Wang BS (2017) Double-layered composite methods extrapolating to complete basis-set limit for the systems involving more than ten heavy atoms: application to the reaction of heptafluoroisobutyronitrile with hydroxyl radical. J Phys Chem A 121:9020–9032
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241
Zhao Y, Truhlar DG (2006) Assessment of model chemistries for noncovalent interactions. J Chem Theory Comput 2:1009–1018
Zhou L, Ravishankara AR, Brown SS, Idir M, Daële V, Mellouki A (2017) Kinetics of the reactions of NO3 radical with methacrylate esters. J Phys Chem A 121:4464–4474
Zhou CW, Li ZR, Li X (2009) Kinetics and mechanism for formation of enols in reaction of hydroxide radical with propene. J Phys Chem A 113:2372–2382
Zhu JQ, Wang SY, Tsona NT, Jiang XT, Wang YF, Ge MF, Du L (2017) Gas-phase reaction of methyl n-propyl ether with OH, NO3, and Cl: kinetics and mechanism. J Phys Chem A 121:6800–6809
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Nos. 21507027, 21707062, and 41775119). The authors acknowledge the Youth Fund Project of HuBei Provincial Department of Education (Q20132501) and Hubei Key Laboratory of Pollutant Analysis & Reuse Technology Open Fund (PA160204).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerhard Lammel
Electronic supplementary material
ESM 1
(DOC 1014 kb)
Rights and permissions
About this article
Cite this article
Chu, H., Wu, W., Shao, Y. et al. A quantum theory investigation on atmospheric oxidation mechanisms of acrylic acid by OH radical and its implication for atmospheric chemistry. Environ Sci Pollut Res 25, 24939–24950 (2018). https://doi.org/10.1007/s11356-018-2561-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2561-6