Abstract
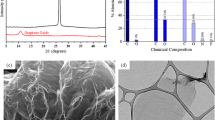
The environmental risks of antibiotics have attracted increasing research attention due to their prevalence and persistence in the aquatic environment. In this study, oxygen functionalized graphene, namely, graphene oxide (GO), was synthesized by modified Hummer’s and Offeman’s method and used as potential effective absorbent for the removal of fluoroquinolones (FQs), i.e., ciprofloxacin (CIP), norfloxacin (NOR), and ofloxacin (OFL), from aqueous solution. The as-synthesized GO was characterized by Fourier transform infrared (FTIR) spectroscopy, powder X-ray diffraction (XRD), thermogravimetric analysis (TGA), and high-resolution transmission electron microscopy (HRTEM). Out of various factors that were taken to consideration while studying the adsorption process, it was found that pH of antibiotic solution is more crucial than the other experimental parameters such as initial antibiotic concentration, contact time, and adsorbent dosage and has significant impact on FQ adsorption via the GO adsorbent. The maximum removal of FQ was observed at pH 7 for CIP and NOR, while adsorption was maximum at pH 4 for OFL. Experimental data best fitted to the pseudo-second-order model as compared to the pseudo-first-order kinetic adsorption model. Best fitting of the equilibrium experimental data to Langmuir isotherm compared to Freundlich isotherm models established that FQ adsorbs over the GO in monolayer manner. Density functional theory (DFT) calculations performed at B3LYP/6-31G(d) level of theory in order to elucidate the thermodynamic feasibility of adsorption process and nature of interactions of antibiotic molecules with the GO adsorbent.

ᅟ
















Similar content being viewed by others
Change history
19 February 2018
Unfortunately, the original version of this article contains a mistake. The figures no. 10, 11, 12 and 13 in the original version of the article should be replaced by the figures shown in this paper.
References
Ai L, Zhang C, Chen Z (2011) Removal of methylene blue from aqueous solution by a solvothermal-synthesizes graphene/magnetic composite. J Hazard Mater 192:1515–1524
Al-Khateeb LA, Almotiry S, Salam MA (2014) Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem Eng J 248:191–199
An T, Yang H, Song W, Li G, Luo H, Cooper WJ (2010) Mechanistic considerations for the advanced oxidation treatment of fluoroquinolone pharmaceutical compounds using TiO2 heterogeneous catalysis. J Phys Chem A 114:2569–2575
Beauvais RA, Alexandratos SD (1998) Polymer-supported reagents for the selective complexation of metal ions: an overview. React Funct Polym 36:113–123
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Berg JM, Tymoczko JL, Stryer L (2002) Chemical bonds in biochemistry, biochemistry, 5th edn. W H Freeman, New York Section 1.3
Bergmann CP, Machado FM (2015) Carbon nanomaterials as adsorbents for environmental and biological applications. Springer, Berlin ISBN-978-3-319-18875-1
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies, some procedures with reduced errors. Mol Phys 19:553–566
Carabineiro SAC, Amornsri TT, Pereira MFR, Serp P, Figueiredo JL (2012) Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotics ciprofloxacin. Catal Today 186:29–34
Chen F, Davidson ER (2002) The effect of the basis set superposition error on the geometry optimization of the p-DFB–N2 complex. Chem Phys Lett 360:99–103
Chen Z, Fu J, Wang M, Wang X, Zhang J, Xu Q (2014) Adsorption of cationic dye (methylene blue) from aqueous solution using poly(cyclotriphosphazene-co-4,4-sulfonyldiphenol) nanospheres. Appl Surf Sci 289:495–501
Chowdhury S, Balasubramanian R (2014) Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv Colloid Interf Sci 204:35–56
Diva RAF, Vasudevan D, Mackay AA (2010) Trends in soil sorption coefficients with in common antimicrobial families. Chemosphere 79:786–793
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–347
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09 (revision C.01) Gaussian. Inc, Wallingford
Gao Y, Li Y, Zhang L, Huang H, Hu J, Shah SM, Su X (2012) Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J Colloid Interface Sci 368:540–546
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F (2013) NBO 6.0, Theoretical Chemistry Institute, University of Wisconsin, MadisonX
Gu C, Karthikeyan KG (2005) Sorption of the antimicrobial ciprofloxacin to aluminium and iron hydrous oxide. Environ Sci Technol 39:9166–9173
Hernández Rosas JJ, Ramírez Gutiérrez RE, Escobedo-Morales A, Anota EC (2011) First principles calculations of the electronic and chemical properties of graphene, graphane, and graphene oxide. J Mol Model 17:1133–1139
Hummers W, Offerman R (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Jauris IM, Matos CF, Saucier C, Lima EC, Zarbin AJG, Fagan SB, Machadod FM, Zanella I (2016) Adsorption of sodium diclofenac on graphene: a combined experimental and theoretical study. Phys Chem Chem Phys 18:1526–1536
Jauris IM, Matos CF, Zarbin AJG, Umpierres CS, Saucier C, Lima C, Fagan SB, Zanella I, Machado FM (2017) Adsorption of anti-inflammatory nimesulide by graphene materials: a combined theoretical and experimental study. Phys Chem Chem Phys 19:22,099–22,110
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, New York
Ji Y, Wang F, Duan L, Zhang F, Gong X (2013) Effect of temperature on the adsorption of sulfanilamide onto aluminum oxide and its molecular dynamics simulations. Appl Surf Sci 285:403–408
Jiang WT, Chang PH, Wang YS, Tsai Y, Jean JS, Li Z, Krukowski K (2013) Removal of ciprofloxacin from water by birnessite. J Hazard Mater 250:362–369
Joakim DGL, Pedro C, Paxeus N (2007) Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J Hazard Mater 148:751–755
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Lee L, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Lerf A, He H, Forster M, Klinowski J (1998) Structure of graphite oxide revisited. J Phys Chem B 102:4477–4482
Li Z, Hong H, Liao L, Ackley CJ, Schulz LA, MacDonald RA, Mihelich AL, Emard SM (2011) A mechanistic study of ciprofloxacin removal by kaolinite. Colloids Surf B 88:339–344
Li M, Huang X, Wu C, Xu H, Jiang P, Tanaka T (2012) Fabrication of two-dimensional hybrid sheets by decorating insulating PANI on reduced graphene oxide for polymer nanocomposites with low dielectric loss and high dielectric constant. J Mater Chem 22:23,477–23,484
Li Y, Du Q, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y (2013a) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem Eng Res Des 91:361–368
Li MJ, Liu CM, Cao HB, Zhang Y (2013b) Surface charge research of graphene oxide, chemically reduced graphene oxide and thermally exfoliated graphene oxide. Adv Mater Res 716:127–131
Li H, Zhang D, Han X, Xing B (2014) Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 95:150–155
Li JR, Wang YX, Wang X, Yuan B, Fu ML (2015) Intercalation and adsorption of ciprofloxacin by layered chalcogenides and kinetics study. J Colloid Interface Sci 453:69–78
Li X, Wang W, Dou J, Gao J, Chen S, Quan X, Zhao H (2016) Dynamic adsorption of ciprofloxacin on carbon nanofibers: quantitative measurement by in situ fluorescence. J Water Process Eng 9:14–20
Lindberg RH, Wennberg P, Johansson MI, Tysklind M, Andersson BAV (2005) Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environ Sci Technol 39:3421–3429
Liu X, Zhang H, Ma Y, Wu X, Meng L, Guao Y, Yu G, Liu Y (2013) Graphene-coated silica as a highly efficient sorbents for residual organophosphorus pesticides in water. J Mater Chem A 1:1875–1884
Lv G, Pearce CW, Gleason A, Liao L, MacWilliams MP, Li Z (2013) Influence of montmorillonite on antimicrobial activity of tetracycline and ciprofloxacin. J Asian Earth Sci 77:281–286
Mackay AA, Serement DE (2008) Probe compounds to quantify cation exchange and complexation interaction of ciprofloxacin with soils. Environ Sci Technol 42:8270–8276
Mallakpour S, Abdolmaleki A, Borandeh S (2014) Covalently functionalized graphene sheets with biocompatible natural amino acids. Appl Surf Sci 307:533–542
Mennucci B (2010) Continuum solvation models: what else can we learn from them? J Phys Chem Lett 1:1666–1674
Michael I, Rizzo L, McArdell CS, Manaia CM, Merlin C, Schwartz T, Dagot C, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: a review. Water Res 47:957–995
Mishra AK, Ramaprabhu S (2011) Functionalized graphene sheet for arsenic removal and desalination of sea water. Deasalination 282:39–45
Najjar NHE, Touffet A, Deborde M, Journel R, Leitner NKV (2013) Levofloxacin oxidation by ozone and hydroxyl radicals: kinetic study, transformation products and toxicity. Chemosphere 93:604–611
Nam SW, Jung C, Li H, Yu M, Flora JR, Boateng LK, Her N, Zoh KD, Yoon Y (2015) Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 136:20–26
Nikolina AT, George ZK, Nikolaos KL, Eleni AD (2013) Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 29:1657–1668
Park HR, Kim TH, Bark KM (2007) Physicochemical properties of quinolone antibiotics in various environments. Eur J Med Chem 37:443–460
Pham TA, Kumar NA, Jeong YT (2010) Covalent functionalization of graphene oxide with polyglycerol and their use as templates for anchoring magnetic nanoparticles. Synth Met 160:2028–2036
Rakshit S, Sarkar D, Elzinga EJ, Punamiya P, Datta R (2013) Mechanisms of ciprofloxacin removal by nano-sized magnetite. J Hazard Mater 246:221–226
Renew JE, Huang C (2004) Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography-electrospray mass spectrometry. J Chromatogr A 1042:113–121
Samuelsen OB (2006) Pharmacokinetics of quinolones in fish: a review. Aquaculture 255:55–75
Schlüsener MP, Bester K (2006) Persistence of antibiotics such as macrolides, tiamulin and salinomycin in soil. Environ Pollut 143:565–571
Seredych M, Bandosz TJ (2007) Removal of ammonia by graphite oxide via its intercalation and reactive adsorption. Carbon 45:2126–2139
Seredych M, Petit C, Tamashausky AV, Bandosz TJ (2009) Role of graphite precursor in the performance of graphite oxides as ammonia adsorbents. Carbon 47:445–456
Seredych M, Tamashausky AV, Bandosz TJ (2010) Graphite oxides obtained from porous graphite: the role of surface chemistry and texture in ammonia retention at ambient conditions. Adv Funct Mater 20:1670–1679
Shujun Y, Xiangxue W, Yuejie A, Xiaoli T, Tasawar H, Wenping H, Xiangke W (2016) Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. J. Mater. Chem. A 4:5654–5662
Singla P, Goel N, Singhal S (2016) Affinity of boron nitride nanomaterials towards antibiotics established by exhaustive experimental and theoretical investigations. Chem Eng J 299:403–414
Sui M, Zhou Y, Sheng L, Duan B (2012) Adsorption of norfloxacin in aqueous solution by Mg–Al layered doubled hydroxides with variable metal composition and interlayer anions. Chem Eng J 210:451–460
Sukul P, Spiteller M (2006) Sulfonamides in the environment as veterinary drugs. Rev Environ Contam Toxicol 187:67–101
Sukul P, Lamshöft M, Kusari S, Zühlke S, Spiteller M (2009) Metabolism and excretion kinetics of 14C-labeled and non-labeled difloxacin in pigs after oral administration, and antimicrobial activity of manure containing difloxacin and its metabolites. Environ Res 109:225–231
Tzeli D, Mavridis A, Xantheas SSJ (2002) First principles examination of the acetylene–water clusters, HCCH-(H2O)x, x = 2, 3, and 4. Phys Chem A 106:11,327–11,337
Van Mourik TJ (2008) Basis set superposition error effects cause the apparent nonexistence of the ethene/benzenium ion complex on the MP2 potential energy surface. Phys Chem A 112:11,017–11,020
Verlicchi P, Galletti A, Petrovic M, Barceló D (2010) Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J Hydrol 389:416–428
Vovusha H, Sanyal S, Sanyal B (2013) Interaction of nucleobases and aromatic amino acids with graphene oxide and graphene flakes. J Phys Chem Lett 4:3710–3718
Wammer KH, Korte AR, Lundeen RA, Sundberga JE, Kristopher M, Arnold WA (2013) Direct photochemistry of three fluoroquinolone antibacterials: norfloxacin, ofloxacin, and enrofloxacin. Water Res 47:439–448
Wang CJ, Li Z, Jiang WT (2011) Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl Clay Sci 53:723–728
Watkinson AJ, Murby EJ, Kolpin DW, Costanzo SD (2009) The occurrence of antibiotics in an urban watershed: from wastewater to drinking water. Sci Total Environ 407:2711–2723
Wieren EMV, Seymour MD, Peterson JW (2012) Interaction of fluoroquinolone antibiotic, ofloxacin with titanium oxide nanoparticles in water: adsorption and breakdown. Sci Total Environ 441:1–9
Wu Q, Li Z, Hong H, Yin K, Tie L (2010) Adsorption and intercalation of ciprofloxacin on montmorillonite. Appl Clay Sci 50:204–211
Xing Z, Sun D, Yu X, Zou J, Zhou W (2014) Treatment of antibiotic fermentation-based pharmaceutical waste water using anaerobic and aerobic moving bed biofilm reactors combined with ozone/hydrogen peroxide process. Environ Prog Sustain 33:170–177
Xu W, Zhang G, Zou SC, Ling ZB, Wang GL, Yan W (2009) A preliminary investigation on the occurrence and distribution of antibiotics in the Yellow River and its tributaries, China. Water Environ Res 81:248–254
Xu J, Wang L, Zhu Y (2012) Decontamination of decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 28:8418–8425
Yang ST, Chen S, Chang Y, Cao A, Liu Y, Wang H (2011) Removal of methylene blue from aqueous solution by graphene oxide. J Colloid Interface Sci 359:24–29
Yu S, Wang X, Ai Y, Tan X, Hayat T, Hu W, Wang X (2016) Experimental and theoretical studies on competitive adsorption of aromatic compounds on reduced graphene oxides. J Mater Chem A 4:5654–5662
Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheet as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10,454–10,462
Acknowledgements
N.G. and S.S. thank the Council of Scientific and Industrial Research (C.S.I.R) via grant no. 01(02833)/15/EMR-II for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
A correction to this article is available online at https://doi.org/10.1007/s11356-018-1381-z.
Electronic supplementary material
ESM 1
(DOCX 141 kb).
Rights and permissions
About this article
Cite this article
Yadav, S., Goel, N., Kumar, V. et al. Removal of fluoroquinolone from aqueous solution using graphene oxide: experimental and computational elucidation. Environ Sci Pollut Res 25, 2942–2957 (2018). https://doi.org/10.1007/s11356-017-0596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0596-8