Abstract
Recent studies identified polychlorinated biphenyl (PCB) sulfate esters as a major product of PCB metabolism. Since hydroxy-PCBs (HO-PCBs), the immediate precursors of PCB sulfates and important contributors to PCB toxicity, were shown to have estrogenic activity, we investigated the estrogenicity/androgenicty of a series of PCB sulfate metabolites. We synthesized the five possible structural sulfate monoester metabolites of PCB 3, a congener shown to be biotransformed to sulfates, a sulfate ester of the paint-specific congener PCB 11, and sulfate monoesters of two HO-PCBs reported to interact with sulfotransferases (PCB 39, no ortho chlorines, and PCB 53, 3 ortho chlorines). We tested these PCB sulfates and 4′-HO-PCB 3 as positive control for estrogenic, androgenic, anti-estrogenic, and anti-androgenic activity in the E- and A-screen with human breast cancer MCF7-derived cells at 100 μM–1 pM concentrations. Only 4′-HO-PCB 3 was highly cytotoxic at 100 μM. We observed structure-activity relationships: compounds with a sulfate group in the chlorine-containing ring of PCB 3 (2PCB 3 and 3PCB 3 sulfate) showed no interaction with the estrogen (ER) and androgen (AR) receptor. The 4′-HO-PCB 3 and its sulfate ester had the highest estrogenic effect, but at 100-fold different concentrations, i.e., 1 and 100 μM, respectively. Four of the PCB sulfates were estrogenic (2′PCB 3, 4′PCB 3, 4′PCB 39, and 4′PCB 53 sulfates; at 100 μM). These sulfates and 3′PCB 3 sulfate also exhibited anti-estrogenic activity, but at nM and pM concentrations. The 4′PCB 3 sulfate (para-para′ substituted) had the strongest androgenic activity, followed by 3′PCB 3, 4′PCB 53, 4PCB11, and 4PCB 39 sulfates and the 4′HO-PCB 3. In contrast, anti-androgenicity was only observed with the two compounds that have the sulfate group in ortho- or meta- position in the second ring (2′PCB 3 and 3′PCB 3 sulfate). No dose–response was observed in any screen, but, with exception of estrogenic activity (only seen at 100 μM), endocrine activity was often displayed at several concentrations and even at 1 pM concentration. These data suggest that sulfation of HO-PCBs is indeed reducing their cytotoxicity and estrogenicity, but may produce other endocrine disruptive activities at very low concentrations.
Similar content being viewed by others
Introduction
Polychlorinated biphenyls (PCBs), a group of 209 individual congeners with different chlorination pattern on a biphenyl core, are a major class of persistent organic pollutants. They bioaccumulate, biomagnify in the food chain, and are ubiquitously detectable in the environment (water, soil, air), wildlife, food, and human tissues (Safe 1993). PCBs were commercially produced from 1928 to the 1980s as mixtures, sold in the USA under the trade name Aroclor, and used in large quantities for a multitude of industrial applications (Robertson and Hansen 2001). An estimated 0.2 million tons of PCBs are distributed worldwide in the environment, and PCBs are still in use in older devices that could leak PCBs into the environment through failure and/or inappropriate disposal (Erickson and Kaley 2011). In addition, new research found that PCBs are currently produced as inadvertent by-products in the manufacture of commercial paints and pigments (Hu and Hornbuckle 2010).
The International Agency for Research on Cancer (IARC) has recently upgraded PCBs to Group 1, human carcinogens (Lauby-Secretan et al. 2013). Moreover, aside from cancer, exposure to PCBs is associated with a broad range of adverse health effects (Silberhorn et al. 1990), including reproductive abnormalities in animal models, developmental toxicity, and endocrine disruptive effects, to mention a few (DeCastro et al. 2006; Ropstad et al. 2006; Safe 1994). Impaired reproduction, changes in behavior, altered fetal and child development, and even promotion of carcinogenesis are often associated with hormonally active agents (Longnecker et al. 1997; National Research Council U.S. 1999; Negri et al. 2003), raising the question whether PCBs or their derivatives are hormonally active compounds.
PCB congeners, especially those possessing few chlorine atoms, are subject to metabolic attack and give rise to a large number of hydroxyl and other metabolites, each with its own biologic activity (Grimm et al. 2015a). Nevertheless, most research to date has focused on commercial PCB mixtures, individual congeners, and to a lesser extent their hydroxylated metabolites. Particularly, the semi-volatile lower chlorinated PCBs that are commonly found in indoor and outdoor air resulting in considerable inhalation exposure (Ampleman et al. 2015; Herrick et al. 2007; Lehmann et al. 2014; Tue et al. 2013), undergo cytochrome P-450 catalyzed oxidative metabolism to mono- and dihydroxy-biphenyls (McLean et al. 1996). Those hydroxylated PCBs are substrates of cytosolic phase II enzymes resulting in glucuronidation and sulfation (Ekuase et al. 2014a; Liu et al. 2009; Sacco et al. 2008; Tampal et al. 2002). Dhakal and coworkers discovered that formation of sulfate conjugates is the major metabolic pathway for PCB 3 in rats in vivo (Dhakal et al. 2012, 2013, 2014). While quite a bit has been learned recently about HO-PCB as substrates and inhibitors of sulfotransferases (Ekuase et al. 2011, 2014b; Liu et al. 2011), very little is known about the potential toxicity and endocrine disruption of PCB sulfate metabolites. However, some HO-PCBs and PCB sulfate metabolites are ligands for human transthyretin which could play a potential role in disruption of thyroid hormone homeostasis (Grimm et al. 2013).
HO-PCBs were reported to elicit estrogenic and anti-estrogenic effects in different test systems (Connor et al. 1997; DeCastro et al. 2006; Gregoraszczuk et al. 2008; Kramer et al. 1997; Krishnan and Safe 1993; Machala et al. 2004; Soto et al. 1995). We hypothesized that sulfation of HO-PCBs would decrease their potential cytotoxic, estrogenic, and androgenic activity. Our objective in this study was to screen a variety of different PCB sulfates over a wide range of concentrations in four different types of endocrine disruption assays to gain a better understanding of the potential toxicity of this kind of metabolite. We used the E- and A-screen in which estrogenic or androgenic activity of a test compound is determined by receptor-mediated induction or inhibition of proliferation of MCF7-BOS or MCF7-AR1 cells, respectively (Soto et al. 1995, 1998; Szelei et al. 1997). We examined the endocrine effects of eight synthetic PCB sulfate monoesters in comparison to a hydroxylated PCB. We report here that some PCB sulfate esters show estrogenic activity, but only at very high concentrations (100 μM), while anti-estrogenic, androgenic, or anti-androgenic activity was more frequently observed and visible at orders of magnitude lower concentrations (pM).
Materials and methods
Chemical substances
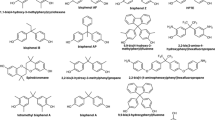
Reagents, media, and media supplements were from Fischer Scientific (Pittsburgh, PA) if not stated otherwise. Beta-estradiol (≥98 % pure) was purchased from MP Biomedicals; Fulvestrant, ICI 182,780 (>98 % pure) and R1881 (≥98 % pure) were purchased from Sigma-Aldrich. The synthesis and characterization of the positive control, 4′-chloro-biphenyl-4-ol (4′-HO-PCB 3), and the ammonium salts of 2′-sulfooxy-4-chloro-biphenyl (2′PCB 3 sulfate), 3′-sulfooxy-4-chloro-biphenyl (3′PCB 3 sulfate), 4′-sulfooxy-4-chloro-biphenyl (4′PCB 3 sulfate), and 4-sulfooxy-3,3′-dichloro-biphenyl (4PCB 11 sulfate) have been described previously (Grimm et al. 2013; Li et al. 2010). Four additional PCB sulfates, including 2-sulfooxy-4-chloro-biphenyl (2PCB 3 sulfate), 3-sulfooxy-4-chloro-biphenyl (3PCB3 sulfate), 4-sulfooxy-3,4′,5-trichloro-biphenyl (4PCB 39 sulfate), and 4-sulfooxy-2,2′5,6′2,2',5',6-tetrachloro-biphenyl (4'PCB 53 sulfate), were synthesized and characterized as described below. The chemical structures and the respective abbreviations of the PCB sulfates and the positive control, 4′-HO-PCB 3, are shown in Fig. 1. All chemicals were dissolved in dimethyl sulfoxide (DMSO) and added to the medium at the indicated concentration. The final concentration of solvent in the culture medium did not exceed 0.5 or 0.6 % v/v of DMSO. Control samples obtained medium containing 0.5 or 0.6 % DMSO without test compound. These concentrations were found to have no effect on cell yields.
Synthesis and characterization of PCB sulfates
General methods
Melting points were measured on a Mel-Temp melting point apparatus and are uncorrected. If not stated otherwise, all PCB sulfates and the corresponding intermediates were characterized by 1H and 13C NMR spectroscopy. The NMR spectra were recorded on a Bruker Avance DRX-400 spectrometer in the University of Iowa Central NMR Research Facility (Iowa City, IA, USA). The NMR samples were prepared in CDCl3 or CD3OD (Cambridge Isotope Laboratories, Andover, MA), and tetramethylsilane (TMS) was used as internal standard. GC-MS analysis of TCE-PCB sulfate diester intermediates was performed in the electron impact (EI) mode on an Agilent 6890N gas chromatograph coupled with an Agilent 5975 Mass Selective Detector (Agilent Technologies, CA, USA) as reported previously (Telu et al. 2010). Only the isotopic ion with the lowest mass is reported for major fragments observed in the MS spectra. Accurate mass determinations of the PCB sulfates were performed by the High Resolution Mass Spectrometry Facility of the University of California Riverside (Riverside, CA, USA). The purity of all PCB sulfates was verified by thin layer chromatography as described previously (Li et al. 2010) prior to preparing DMSO stock solutions for cell culture experiments to ensure that no hydrolysis to the corresponding OH-PCB had occurred.
Characterization of TCE-protected PCB sulfate diesters
Sulfuric acid 4-chlorobiphenyl-2-yl 2,2,2-trichloroethyl sulfate
Reaction of 4-chloro-biphenyl-2-ol (300 mg) (Zhai et al. 2011) and 2,2,2-trichloroethyl chlorosulfate (441 mg) in the presence of Et3N/DMAP in THF yielded 300 mg (49 %) of the product as a colorless oil (Li et al. 2010). 1H NMR (400 MHz, CDCl3): δ/ppm 3.92 (s, 2H), 7.37–7.41 (m, 2H), 7.42–7.45 (m, 2H), 7.46–7.47 (m, 2H), 7.48–7.51 (m, 1H), 7.57 (d, 1H, J = 8.0 Hz); 13C NMR (100 MHz, CDCl3): δ/ppm 79.9, 92.3, 123.1, 128.6, 128.7, 129.0, 129.6, 132.3, 133.6, 134.4, 135.4, 146.9. GC-MS m/z (relative abundance, %): 416(18), 284(10), 204(100), 175(36), 139(32).
Sulfuric acid 4-chlorobiphenyl-3-yl 2,2,2-trichloroethyl sulfate
Reaction of 4-chloro-biphenyl-3-ol (400 mg) (Zhai et al. 2011) and 2,2,2-trichloroethyl chlorosulfate (497 mg) in the presence of Et3N/DMAP in THF yielded 550 mg (80 %) of the product as a colorless oil (Li et al. 2010). 1H NMR (400 MHz, CDCl3): δ/ppm 4.97 (s, 2H), 7.39–7.41 (m, 1H), 7.43–7.46 (m, 2H), 7.49–7.55 (m, 4H), 7.74 (d, 1H, J = 8.0 Hz); 13C NMR (100 MHz, CDCl3): δ/ppm 80.8, 92.4, 121.6, 125.5, 127.1, 127.4, 128.6, 129.3, 131.4, 138.4, 142.2, 146.2. GC-MS m/z (relative abundance, %): 416(16), 284(13), 204(51), 168(100), 139(38).
Sulfuric acid 3,4′,5-trichlorobiphenyl-4-yl 2,2,2-trichloroethyl sulfate
Reaction of 3,4′,5-trichloro-biphenyl-4-ol (160 mg) (Joshi et al. 2011) and 2,2,2-trichloroethyl chlorosulfate (175 mg) in the presence of Et3N/DMAP in THF yielded 220 mg (78 %) of the product as a white solid (Li et al. 2010), mp: 117–118 °C. 1H NMR (400 MHz, CDCl3): δ/ppm 5.04 (s, 2H), 7.43 (s, 4H), 7.55(s, 2H). 13C NMR (100 MHz, CDCl3): δ/ppm 80.4, 92.4, 127.9, 128.5, 129.6, 129.9, 135.4, 135.8, 141.4. GC-MS m/z (relative abundance, %): 484(11), 273(100), 243(21), 207(11), 138(32), 61(18).
Characterization of PCB sulfate monesters, ammonium salts
2-Sulfooxy-4-chloro-biphenyl, ammonium salt (2PCB 3 sulfate)
Deprotection of sulfuric acid 4-chlorobiphenyl-2-yl 2,2,2-trichloroethyl sulfate (250 mg) with zinc powder-ammonium formate in ethanol yielded the desired ammonium salt (165 mg; 92 %) as a white solid (Li et al. 2010), mp: 99 °C (dec.); 1H NMR (400 MHz, CD3OD): δ/ppm 7.22 (dd, 1H, J = 4.0 Hz, J = 8.0 Hz), 7.31-7.41 (m, 4H), 7.57 (AA′XX″ system, 2H), 7.78 (d, 1H, J = 2.0 Hz); 13C NMR (100 MHz, CD3OD): δ/ppm 122.4, 125.7, 128.3, 129.1, 130.6, 132.8, 134.1, 138.5, 151.6; HRMS (ESI, negative): [M-NH4]− found m/z 282.9832, calculated for C12H8 35ClO4S 282.9837.
3-Sulfooxy-4-chloro-biphenyl, ammonium salt (3PCB 3 sulfate)
Deprotection of sulfuric acid 4-chlorobiphenyl-3-yl 2,2,2-trichloroethyl sulfate (474 mg) with zinc powder-ammonium formate in ethanol yielded the desired ammonium salt (320 mg; 93 %) as a white solid (Li et al. 2010), mp: 85 °C (dec.); 1H NMR (400 MHz, CD3OD): δ/ppm 7.33–7.45 (m, 4H), 7.47 (d, 1H, J = 8.4 Hz), 7.62 (AA′XX′ system, 2H), 7.89 (d, 1H, J = 2.4 Hz); 13C NMR (100 MHz, CD3OD): δ/ppm 122.2, 125.0, 126.7, 127.9, 128.8, 130.0, 131.4, 140.8, 142.2, 150.2; HRMS (ESI, negative): [M-NH4]− found m/z 282.9840, calculated for C12H8 35ClO4S 282.9837.
4-Sulfooxy-3,4′,5-trichloro-biphenyl, ammonium salt (4PCB 39 sulfate)
Deprotection of sulfuric acid 3,4′,5-trichlorobiphenyl-4-yl 2,2,2-trichloroethyl sulfate (170 mg) with zinc powder-ammonium formate in ethanol yielded the desired ammonium salt (70 mg; 54 %) as a white solid (Li et al. 2010), mp: 150 °C (dec.); 1H NMR (400 MHz, CD3OD): δ/ppm 7.45 (AA′XX ′ system, 2H), 7.58 (AA′XX′ system, 2H), 7.62 (s, 2H); 13C NMR (100 MHz, CD3OD): δ/ppm 128.2, 129.5, 130.2, 132.1, 135.4, 138.1, 139.5, 146.7; HRMS (ESI, negative): [M-NH4]− found m/z 350.9049, calculated for C12H6 35Cl3O4S 350.9058.
4-Sulfooxy-2,2′,5′,6-tetrachloro-biphenyl, ammonium salt (4'PCB 53 sulfate)
Reaction of 2,2′,5′,6-tetrachloro-biphenyl-4-ol (210 mg) (Joshi et al. 2011) and 2,2,2-trichloroethyl chlorosulfate (203 mg) in the presence of Et3N/DMAP in THF yielded 320 mg (90 %) of crude 2,2′,5′,6-tetrachloro-biphenyl-4-yl 2,2,2-trichloroethyl sulfate as a colorless oil. Subsequent deprotection of the crude TCE-protected PCB sulfate (300 mg) with zinc powder-ammonium formate in ethanol yielded the desired ammonium salt (190 mg; 81 %) as a white solid (Li et al. 2010), mp: 140 °C (dec.); 1H NMR (400 MHz, CD3OD): δ/ppm 7.29 (d, 1H, J = 2.4 Hz), 7.45 (dd, 1H, J = 2.4 Hz, J = 8.4 Hz), 7.66 (s, 1H), 7.53 (d, 1H, J = 8.4 Hz); 13C NMR (100 MHz, CD3OD): δ/ppm 121.9, 131.2, 131.9, 132.3, 133.2, 133.7, 133.8, 135.9, 138.6, 154.8; HRMS (ESI, negative): [M-NH4]− found m/z 386.8637, calculated for C12H5 35Cl4O4S 384.8668.
MCF7 cell culture
The estrogen-sensitive human breast cancer cell lines MCF-7 BOS and MCF7-AR1 were kindly provided by Drs. Ana M. Soto and Carlos Sonnenschein (Tufts University School of Medicine, Department of Anatomy and Cellular Biology, Boston, Massachusetts). For maintenance, cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (DMEM/10 %FBS), at 37 °C with 6 % CO2 in a humidified incubator as described by DeCastro and coworkers (DeCastro et al. 2006).
Cytotoxicity measurement with resazurin
To examine cell viability, 5 × 103 MCF-7 BOS cells were seeded in 100-μl DMEM/10 %FBS per well in 96-well plates. After 24 h, cells received fresh medium containing test compounds at a range from 1 pM or 5 nM to 100 μM or solvent alone (DMSO, final concentration 0.5 % in medium). Normal medium and FCS were used in this assay to enable the assessment of cytotoxic and proliferation inhibiting activity of the test compounds, since these cells do hardly proliferate in medium with charcoal-treated FBS as used below in the endocrine disruption assay. After 120 h exposure, the medium was removed, cells were washed once with phosphate-buffered saline (PBS) and incubated in phenol red-free DMEM/10 %FBS containing resazurin (50 μM) for 1 h. The assay measures the reduction of non-fluorescent resazurin to fluorescent resorufin by viable, metabolically active cells. The rate of fluorescence is directly proportional to the number of viable cells per well. The fluorescence was measured with a Tecan Genios Pro plate reader at 535-nm excitation and 590-nm emission wavelengths.
E-screen with MCF7-BOS cells to test estrogenicity and anti-estrogenicity
Estrogenicity of a test compound is assessed in the E-screen assay by measuring compound-induced proliferation of MCF-7 BOS human breast cancer cells in estrogen-free medium. These cells respond to estrogenic compounds that bind to the cytoplasmic estrogen receptor (ER) with stimulated cell proliferation. According to a technique slightly modified from Soto et al. (1995), 3 × 104 MCF7-BOS cells per well in 1-ml DMEM/10 %FBS were seeded into 24-well plates. A 48 h attachment time was used to obtain good adherence of the cells to the well surface. The cells were then carefully washed with phenol red-free DMEM supplemented with 5 % charcoal-dextran-treated FBS (DMEM/5 %CD-FBS) and then incubated in duplicate for each data point in this DMEM/5 %CD-FBS with test compounds (concentration range of 1 pM–100 μM) or 0.5 % DMSO (hormone-free negative control) or estradiol (E2, 100 pM, positive control). After 120-h incubation, the wells were checked visually to assure that the samples were subconfluent, the medium was removed, and the sulforhodamine B (SRB) protein assay was used to evaluate cell growth. Briefly, cells were fixed onto the plates with cold 10 % trichloroacetic acid for 30 min at 6 °C, then washed 3 times with water, and afterwards air-dried. Cells were stained with 0.4 % SRB in a 1 % acetic acid solution for 20 min at room temperature and afterwards washed with 1 % acetic acid until the wash solution in the plate was clear. After air-drying of the fixed and stained cells, exactly 600μl Tris base buffer (10 mM) was added to each well, the plates were shaken rapidly for at least 10 min until the color was extracted from the cells, and a homogeneous solution was visible. Absorption of the solution was measured at 540 nm using a microtiter plate reader (Tecan Genios Pro) which is proportional to the cell number/protein content per well. To confirm that the proliferation induction was ER mediated, parallel cultures were prepared and co-exposed to the ER antagonist ICI 182,780 (10 nM in 0.1 % DMSO) and the test compound which should give negative results.
The estrogenic activity of xenobiotics was assessed by calculating the relative estrogenic proliferative effect (REE), which is 100 times the ratio of the highest proliferative effect (PE) obtained with the chemical (compound PE-1) compared to 100 pM E2 (E2 PE-1), i.e., the formula used is as follows: 100 × compound PE-1/E2 PE-1. The REE compares quantitatively the magnitude of the proliferative response of the test compound, the efficacy of the compound compared to the one of E2. This response is then categorized as follows: REE = 80–100 % as full agonist, 25–80 % as partial agonist, 10–25 % as week agonist, and ≤10 % as negative result. Also calculated were the relative estrogenic proliferative potency (RPP) which is the ratio between the minimal concentration of E2 needed for maximal cell yield and the minimal concentration of the test compound needed to achieve a similar effect times 100.
Anti-estrogenicity: The E-screen can also assess anti-estrogenicity by inducing the maximal proliferation of MCF7-BOS cells with E2 and measuring the reduction in proliferation that occurs as a result of co-incubation with the test compound. Cells were seeded into 24-well plates and 48 h later washed with phenol red-free DMEM/5 %CD-FBS as described above and then treated in duplicate with E2 (100 pM in 0.1 % DMSO) in the presence of different concentrations (1 pM–100 μM) of the test compound or 0.5 % DMSO. After 5-day exposure, the SRB measurement was carried out as described above. A reduction of the stimulated cell proliferation effect from E2 indicates an anti-estrogenic activity.
A-screen with MCF7-AR1 cells to test androgenicity and anti-androgenicity
Androgenicity was examined with MCF7-AR1 cells which are MCF7-BOS cells stably transfected with the human androgen receptor (AR). If a test compound acts as androgen, it induces an AR-mediated proliferative shutoff and reduces the proliferation induced through E2. According to a technique slightly modified from Soto et al. (1998), 5 × 104 MCF7-AR1 cells were seeded into 24-well plate in 1ml DMEM/10 %FBS. This little higher seeding cell number compared to the MSC7-BOS cells was used to compensate for the slightly slower proliferation and attachment rate of the MCF7-AR1 cells. After 48 h attachment time, cells were carefully washed with phenol red-free DMEM/5 %CD-FBS and then co-treated in duplicate with different concentrations (1 pM–100 μM) of the test compound or 0.5 % DMSO and E2 (100 pM in 0.1 % DMSO). After 120 h, the assay was stopped by removing the medium from the wells, and SRB measurement was carried out.
The relative androgenic effect (RAE) was assessed by calculating a ratio from the reduced cell yield of R1881 (500 pM), the androgen “gold standard,” and the most significant reduced cell yield from the test compound times 100.
Anti-androgenicity: 5 × 104 MCF7-BOS cells were seeded as described above and 48 h later washed with phenol red-free DMEM/5 %CD-FBS and then co-treated with different concentrations (100 μM–1 pM) of the test compound and E2 (100 pM in 0.05 % DMSO) and R1881 (10 μM in 0.05 % DMSO), an androgen receptor agonist. After 120 h, the assay was stopped, and SRB measurement was carried out. If the test compound blocks the androgen-induced inhibition of cell proliferation by R1881 and stimulates cell proliferation, it acts as an anti-androgen.
Statistics
Experiments were performed in duplicate, and every experiment was repeated at least once. The test compounds evaluated with the E-screen assay were considered estrogenic if it induced a statistically significant increase in cell yield over the hormone-free control. Results were normalized to steroid-free control 1 % (for estrogenicity), E2 (anti-estrogenicity and androgenicity), or E2 + R1881 (anti-androgenicity) and expressed as the mean ± standard error (SE). Differences between treatments were analyzed statistically by using one-way ANOVA with Dunnett’s test at the 5 % level. Levels of significance are indicated as follows: *p < 0.05; **p < 0.01, and ***p < 0.001.
Results
Synthesis and characterization of PCB sulfates
Eight PCB sulfates, including five sulfate esters of HO-PCB 3, were selected to investigate their estrogenicity and androgenicity in vitro (Fig. 1). Four new PCB sulfates, including 2PCB 3 sulfate, 3PCB 3 sulfate, 4PCB 39 sulfate, and 4′PCB 53 sulfate, were synthesized as the respective ammonium salts using a published approach (Grimm et al. 2013; Li et al. 2010) and fully characterized (see “Materials and methods”). This approach allows the preparation of milligram quantities of PCB sulfates in high purity (>99 %) as determined using thin layer chromatography (Li et al. 2010) and HPLC (Grimm et al. 2013). Briefly, the corresponding HO-PCB was reacted with 2,2,2-trichloroethyl chlorosulfate (Hedayatullah et al. 1972) to yield a 2,2,2-trichloroethyl (TCE-) PCB sulfate diester. Deprotection with zinc powder-ammonium formate yielded the ammonium salt of the corresponding PCB sulfate. The synthesis and characterization of the other four PCB sulfates have been reported earlier (Grimm et al. 2013; Li et al. 2010). The ammonium salts of all eight PCB sulfates are colorless solids and well soluble in DMSO. PCB sulfates can be stored both as neat compound and solution in DMSO for several months without detectable degradation to the corresponding HO-PCBs.
Cytotoxicity/cell viability
To establish the experimental concentration range for the cell proliferation assays, the cytotoxicity of PCB sulfates was determined at concentrations from 5 nM or 1 pM to 100 μM after 120 h (5 days) of exposure in DMEM/10 %FBS medium. The percent of life cells compared to solvent-treated controls was quantified using the resazurin assay, an assay based on metabolic activity of living cells. Cells were also visually inspected using an inverted microscope to evaluate viability, cell number, and morphology of the cells. One compound, 4′-HO-PCB 3, was 100 % toxic at 100 μM, and this concentration was therefore not used in the E- and A-screen assay. Also, 4′PCB 53 sulfate was weakly cytotoxic after 120 h. Since the reduction of viable cells was only 16 % compared to the control, this concentration was included in the E- and A-screen (Supplementary Material, Fig. S1). No significant cytotoxicity was observed with any of the other PCB sulfates up to concentrations of 100 μM.
E-screen for estrogenic and anti-estrogenic activity
The MCF7-BOS cell line was used to measure estrogenicity/induction of cell proliferation in estrogen-free medium. 4′-HO-PCB 3, used as a positive PCB control, was the most potent of all tested PCB metabolites, inducing significant cell proliferation at 1 μM concentration (RPP of 0.1 %; Fig. 2 and Table 1). Four of the eight tested PCB sulfate monoesters also showed estrogenic activity but only at the highest concentration tested, 100 μM, equivalent to an RPP of 0.001 % (Fig. 2 and Table 1). Of these, the 4′PCB 3 sulfate had the highest estrogenic efficacy, surpassing the effect seen with its parent compound 4′-HO-PCB 3 at 1 μM, and reaching that of E2, although only at a 6 orders of magnitude higher concentration (100 μM vs 100 pM, respectively, RPP of 0.001 % and highest REE of 78 %). The other sulfate esters followed in the order 2′PCB 3 sulfate > 4PCB 39 sulfate > 4′PCB 53 sulfate at 100 μM. The addition of an ER antagonist ICI 182,780 in parallel cultures confirmed that these inductions of proliferation were mediated through ER activation since ICI 182,780 completely blocked the effect (Fig. S4). The other tested sulfate esters, 2PCB 3 sulfate, 3PCB 3 sulfate, 3′PCB 3 sulfate and 4PCB 11 sulfate, did not show any estrogenicity at any concentration investigated, i.e., no higher cell proliferation compared to DMSO controls (Supplementary Material, Fig. S2).
Five of the eight PCB sulfate monoesters showed anti-estrogenic activity by reducing the E2-stimulated cell proliferation (Fig. 3). 4′PCB 3 sulfate was anti-estrogenic at almost all, i.e., 4 out of 5 concentrations tested, ranging from 1 pM up to 100 μM. Similarly, 2′PCB 3 sulfate and 3′PCB 3 sulfate were anti-estrogenic at 3 of 5 concentrations tested, particularly at the lower ones. 4PCB 39 sulfate was only active at 1 pM and 4′PCB 53 sulfate only at 10 nM and 100 μM concentrations. 2PCB 3 sulfate, 3PCB 3 sulfate, and 4PCB 11 sulfate did not cause any significant reduction of cell proliferation compared to E2 controls at the tested concentrations, neither did 4′-HO-PCB 3 (Supplemental Material, Fig. S2).
A-screen for androgenicity and anti-androgenicity
Five out of eight tested PCB sulfate monoesters had androgenic effects over a wide range of concentrations (some from 1 pM to 100 μM). 4′PCB 53 sulfate at 100 μM had the highest androgenic efficacy with a RAE of 70 %, followed by 4′PCB 3 sulfate (100 μM; RAE of 60 %, Fig. 4 and Table 1). 4′PCB 3 sulfate and 3′PCB 3 sulfate had the highest potency, exhibiting significant androgenic activity at a concentration of 1 pM. Both compounds were androgenic at 4 of the 5 concentrations tested over a range of 1 pM to 100 μM. 4PCB 11 and 4PCB 39 were less androgenic, each at 2 of the concentrations in the middle range of concentrations tested with a RAE of 51 and 48 %, respectively. This qualifies these 4 PCB sulfates as partial agonists. In contrast, 2′PCB 3 sulfate, 2PCB 3 sulfate, and 3PCB 3 sulfate did not show any androgenicity, i.e., they did not lower E2-induced cell proliferation at any tested concentration (Supplemental Material, Fig. S3).
Only two (2′PCB 3 and 3′PCB 3 sulfate) of the eight PCB sulfate monoesters showed weak anti-androgenic activity, i.e., higher cell proliferation compared to the E2 + R1881, and both had this effect at very low concentrations (100 and 1 pM, Fig. 5). All other seven compounds did not increase cell proliferation at the concentrations tested (Supplementary Material, Fig. S3).
Comparison of ED effects of all tested compounds
These newly synthesized PCB sulfate monoesters are not cytotoxic up to 100 μM concentration in MCF-7 cells. Table 1 provides a summary of calculations for relative estrogenic effect/efficacy (REE), relative estrogenic proliferative potency (RPP), and relative androgenic effect/efficacy at the concentrations with the most significant effect. Two compounds (2PCB 3 sulfate and 3PCB 3 sulfate) did not act as disruptor in any of the screens. While 4′-HO-PCB 3 acted as potent estrogen with a RPP of 0.1 % and REE of 60 % at 1 μM concentration, some sulfates like the 4′PCB 3 sulfate acted like estrogens only at very high concentrations (100 μM) with an RPP of 0.001 % and REE of 78 %. Anti-androgenicity was restricted to 2 sulfates while 5 sulfates had anti-estrogenic and/or androgenic activity. We saw the most significant effect of androgenic activity with the 4′PCB 53 sulfate at 100 μM with a RAE of 70 %. 4′-HO-PCB 3 also displayed androgenic activity at two concentrations, and the most significant one at 100 μM resulted in an RAE of 55 %. However, with most compounds, no classical dose–response curves (increasing effect with increasing concentration) were seen in our experiments. These preliminary data suggest that these PCB sulfate monoesters have low cytotoxic potential in human breast cancer cells as well as low potential to act as endocrine disrupting agent.
Discussion
PCBs are ubiquitous human and environmental contaminants and cause a multitude of adverse health effects, but the mechanisms of action are often not known. Lower chlorinated PCBs undergo oxidative metabolism to hydroxylated PCBs which are important contributors to PCB toxicity (Grimm et al. 2015a; Ludewig et al. 2008). These metabolites are substrates of cytosolic phase II enzymes. Using a rat model, Dhakal and co-workers recently discovered that sulfation is a major phase II pathway, that sulfate metabolites of PCB 3 were detected in blood and urine even after low-dose inhalation exposure, and that the concentration of PCB 3 sulfates exceeds those of their respective HO-PCBs in serum in vivo (Dhakal et al. 2012, 2013, 2014). Also, a new study showed that the sulfated metabolites of PCB 3 and PCB 11 are high affinity ligands of transthyretin in vitro and could play a role in Alzheimer disease as inhibitors of transthyretin fibrillogenesis (Grimm et al. 2013, 2015b). Very little is known about this dominant class of PCB metabolites and their toxicity. To gain better knowledge about their cytotoxicity and potential biological activity as hormone mimics, we synthesized and evaluated all possible mono-sulfates of PCB 3, a sulfate metabolite of PCB 11, a non-Aroclor congener discovered in high levels in paint, indoor and outdoor air, and human blood (Hu et al. 2008; Marek et al. 2013), and one congener each with 3 and 4 chlorines in specific positions, PCB 39 with no ortho chlorines and PCB 53 with 3 ortho chlorines, respectively (Hu and Hornbuckle 2010). To our knowledge, this is the first study examining the potential of PCB sulfates for (anti-)estrogenic and (anti-)androgenic activities and cytotoxicity in cells in culture.
In recent years, global concerns have been raised that about 10 % of man-made chemicals could affect the endocrine system even though only a handful of endocrine disruptors have been confirmed so far (Bergman et al. 2013). Human and wildlife health depends on the ability to reproduce and develop normally, and endocrine disruptors were specifically considered the new theme of the United Nations Integrated Management of Chemicals 2012 initiative and are the emphasis of the US EPA Endocrine Disruptor Screening Program. Among the ED test systems, the E-screen and A-screen are simple and stable assays to determine the activity of compounds to interact with the ER and AR steroid hormone receptors (Soto et al. 1995; Szelei et al. 1997). We used these assays to gain first insight into the endocrine disrupting activity of PCB sulfate metabolites.
The cytotoxicity prescreening showed that only 4′-HO-PCB 3, not its 4′PCB 3 sulfate metabolite or any of the other 6 PCB sulfate metabolites tested, had significant cytotoxic effects in MCF7-BOS cells after 120 h of exposure. This indicates that the introduction of a sulfate group strongly reduces the cytotoxicity of the hydroxylated parent compound. This conclusion is further supported by the results of Machala et al. who reported a cytotoxicity LOEC of 50 μM for 4′-HO-PCB 3 and 20 μM for 2′- and 3′-HO-PCB 3 in T47D.Luc cells (Machala et al. 2004), while we did not see any toxicity up to 100 μM with the sulfate derivatives of these compounds in our assay. Thus, phase II metabolism is a true detoxification mechanism for these HO-PCBs with respect to cytotoxicity.
A recent study of 20 PCB congeners employing an ER dual-luciferase reporter gene assay reported that PCB 18, 28, 49, 52, 99, 101, 103, 110, and 128 exhibited estrogenic effects, whereas PCB 118, 138, 163, 170, 180, 187, 194, 199, and 203 behaved as anti-estrogens and PCB 30 and 44 had both effects (Zhang et al. 2014). This infers that less chlorinated PCBs behave estrogenic while higher chlorinated ones behave as anti-estrogens. Machala and coworkers reported significant ER-mediated activity with hydroxylated low molecular weight PCBs like 3′-HO-PCB3 and 4′-HO-PCB 3 in human mammary cell lines MVLN and T47D.Luc transfected with a luciferase reporter gene. They screened 16 mono-, 10 di-hydroxy, and 6 quinone metabolites of PCBs with 1 to 5 chlorine substituents. Only mono-hydroxylated PCBs with 1 or 2 chlorines, one 3,4-diOH congener, and 4′-HO-PCB 34 had strong estrogenic activity, displayed at low μM concentrations in both cell lines (Machala et al. 2004). This is in agreement with our observation of an estrogenic effect with 4′-HO-PCB3 at 1 μM concentration and a REE of 60 % and RPP of 0.1 %. We now could show that sulfation strongly reduced the efficacy of these metabolites as estrogens. Machala and coworkers also reported that HO-PCBs had significantly higher estrogenic potencies in MVLN and T47D.Luc cells than E2 and they acted in an additive way with E2 (Machala et al. 2004). Of our sulfate derivatives, only 4′PCB 3 sulfate produced an estrogenic effect with a REE of 78 % and RPP of 0.001 % that came close to the activity of E2 (a REE of 80 % is considered a full agonist). This sulfate also had an anti-estrogenic effect in the presence of E2. Machala et al. reported anti-estrogenic activity with most mono- and di-hydroxylated metabolites, but only at concentrations in the cytotoxic range (Machala et al. 2004). Connors and coworkers used a battery of assays to examine estrogenic/anti-estrogenic activity of OH-PCBs and found that most compounds bound to the ER with high affinity, but only few activated the ER while many inhibited ER activation (Connor et al. 1997). We observed anti-estrogenic activity with 5 of 8 sulfate metabolites of PCBs. These findings suggest that while hydroxylation increases estrogenicity of PCBs, sulfation of these hydroxyl-metabolites decreases the estrogenic activity but increases/creates an anti-estrogenic activity. It should also be emphasized that 4 of the sulfates are anti-estrogenic at very low, nano- and pico-molar concentrations and estrogenic only at the highest concentration tested (100 μM), suggesting that the anti-estrogenicity should be the major concern in vivo.
Very few studies examined the effects of PCBs and their metabolites on the androgen receptor. Endo and coworkers reported concentration-dependent anti-androgenic activity of two dioxin-like congeners, PCB77 and PCB126, in LNCap prostate cancer cells (Endo et al. 2003). The ortho-substituted PCB118 and PCB153 had a biphasic effect, stimulated cell proliferation at low and reduced proliferation at high concentrations. The authors suggest both AR- and AhR-mediated pathways as well as modulation of post-transcriptional targets as mechanisms. The PCB mixtures Delor 103 and Aroclor 1254 activated the human AR in a reporter assay in vitro (Portigal et al. 2002; Svobodova et al. 2009), while Aroclor mixtures (1248 > 1254 > 1242 > 1260) and the congeners PCB 42, 128, and 138, but not PCB 31, 99, 118, 153, 168, 180, or 198 acted as anti-androgens mostly at μM concentrations (Bonefeld-Jorgensen et al. 2001; Portigal et al. 2002; Sperry and Thomas 1999). Fish have two androgen receptors and PCB mixtures, Aroclor 1254 > 1260, and hydroxylated PCB congeners displaced DHT from AR2 at medium to high μM concentrations (Sperry and Thomas 1999). We observed androgenic activity with the 4′-HO-PCB 3 (RAE of 55 %) and five sulfate metabolites and anti-androgenicity with two PCB sulfates, with most of these effects visible in the pico- or nanomolar range. Unlike the interaction with the ER, only one compound, 3′PCB 3 sulfate, both activated and inhibited the AR and both reactions occurred at the same picomolar concentration. Overall, it appears that many PCBs have anti-androgenic activity whereas HO-PCBs and their sulfate metabolites are more prone to be activators of the androgen receptor.
Six of eight compounds tested showed significant androgenic and/or anti-estrogenic/androgenic activity at more than one concentration tested, but the effect was not dose-dependent, often spanning several orders of magnitude and starting at a picomolar concentration, suggesting that these compounds could interfere with the endocrine system at very low concentrations. Endocrine-disrupting chemicals may display U-shaped or inverted U-shaped non-monotonic dose–response curves, and it was reported that they rarely exhibit a clear dose–response (Vandenberg et al. 2012; Zoeller and Vandenberg 2015). This weak, constant effect instead of the more common dose–response may be due to specificities of the assay that limit the anti-estrogenic and androgenic effect to a range between no-effect to estrogen-induced level.
We were interested in structure-activity relationships (SAR). Since only a limited number of compounds could be synthesized and screened, we decided to examine (1) the effect of the 5 possible different positions of the sulfate group on the PCB 3 molecule and (2) the effect of non-ortho (PCB 39) vs ortho-chlorinated (PCB 53) and (3) para- (PCB 3, 39) vs meta-chlorinated (PCB 11, 53) metabolites with the sulfate in para position. Overall, some interesting SARs emerged from these studies that need further examination. The position of the sulfate group on the PCB 3 molecule had a strong influence. If it was in the same ring with the chlorine group, it abolished any interaction with the ER and AR (2PCB 3 and 3PCB 3 sulfate). Compounds with the sulfate group in the other ring were active in 3 of the 4 endpoints, with 3′PCB 3 sulfate being not estrogenic, 2′PCB 3 sulfate not androgenic, and 4′PCB 3 sulfate not anti-estrogenic. This latter compound was also the most potent estrogenic compound of all sulfates tested, suggesting that the long para-to-para alignment of the substitutions favors interactions with the ER. Unexpectedly and opposite to findings with pure PCB congeners, non-ortho vs ortho-chlorination did not have an influence in these assays, while the one compound with only meta-chlorination (4PCB 11) only showed some androgenicity. It is of interest that the only anti-androgenic compounds (2′PCB 3, 3′PCB 3) are the PCB 3′s with sulfates in the opposite ring in ortho- and meta- position.
The ER and AR are very similar in structure. The C-terminal region contains the ligand binding domain. This region consists of 11 α-helices that form the barrel-like hydrophobic pocket of the binding cavity. A 12th α-helix at the extreme end of the C-terminus blocks the coactivator binding site, but changes the position to “cap” the lid over the barrel after binding of a ligand, thereby exposing the coactivator binding site (Pike et al. 1999, 2000; Portigal et al. 2002). The binding pocket can accept a large number of compounds with those that fit only loosely acting as weak activators while those that have portions of their structure protruding over the edge acting as antagonists (Eick et al. 2012; White et al. 1994). It can be speculated that having a hydrophilic sulfate in the chlorinated ring (2PCB 3 and 3PCB 3 sulfates) may prevent entry into the barrel-like structure of the ER and AR, thereby preventing any interaction with these receptors. Ortho- and meta- sulfate in the opposite ring may be hanging over the lid of the barrel, thereby hindering the closure and activation of the ER (3′PCB 3 sulfate) or AR (2′PCB 3 sulfate) receptor.
A negative correlation was observed between PCBs and sperm motility in fish (Jenkins et al. 2014), a positive correlation between PCBs and abnormal phenotypic sex differentiation in rodents (Gray and Kelce 1996) and cryptorchidism in boys (Brucker-Davis et al. 2008), an inverse correlation between PCBs and blood testosterone levels in humans and polar bears (Goncharov et al. 2009; Oskam et al. 2003), and a positive correlation between PCBs and feminization of behavior in male mice (Fanini et al. 1990). This very partial list of PCB effects on development and reproduction indicate that PCBs as a group have endocrine disrupting activity. However, our results suggest that this activity may, at least in part, be mediated by their metabolites, including the sulfate esters and that the type of endocrine activity, estrogenic, androgenic, anti-estrogenic, anti-androgenic, or no activity, depends on the metabolites formed. This supports the hypothesis that the specificity of the steroid hormone receptors action is a consequence of endogenous metabolic activity (Lathe and Kotelevtsev 2014).
Conclusions
Our results show that the sulfate metabolites of PCBs are less toxic and have less estrogenic activity than their hydroxylated progenitors, but that they may have anti-estrogenic and androgenic activity at very low concentrations. Thus, it is not clear whether sulfation is a true detoxification or possibly an activation pathway, at least with respect to endocrine activity. Sulfate metabolites appear to be the major product of phase II metabolism of HO-PCBs, and even though their endocrine activity was mostly relatively small, it occurred at very low concentrations. PCB 3 sulfates were readily detectable in blood and urine of rats even after low-dose inhalation exposure (Dhakal et al. 2014) and were also identified in poplar trees (Zhai et al. 2013); however, it is currently unclear if PCB sulfates are also major PCB metabolites in humans. This suggests that more investigations into their occurrence and effects are warranted.
References
Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS (2015) Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol 49:1156--1164
Bergman A et al (2013) The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect 121:A104–A106
Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM (2001) Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology 158:141–153
Brucker-Davis F, Wagner-Mahler K, Delattre I, Ducot B, Ferrari P, Bongain A, Kurzenne JY, Mas JC, Fenichel P, Cryptorchidism Study Group from Nice A (2008) Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum Reprod 23:1708–1718
Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P (1997) Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure-activity relationships. Toxicol Appl Pharmacol 145:111–123
DeCastro BR, Korrick SA, Spengler JD, Soto AM (2006) Estrogenic activity of polychlorinated biphenyls present in human tissue and the environment. Environ Sci Technol 40:2819–2825
Dhakal K, He X, Lehmler HJ, Teesch LM, Duffel MW, Robertson LW (2012) Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem Res Toxicol 25:2796–2804
Dhakal K, Adamcakova-Dodd A, Lehmler HJ, Thorne PS, Robertson LW (2013) Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3). Chem Res Toxicol 26:853–855
Dhakal K, Uwimana E, Adamcakova-Dodd A, Thorne PS, Lehmler HJ, Robertson LW (2014) Disposition of phenolic and sulfated metabolites after inhalation exposure to 4-chlorobiphenyl (PCB3) in female rats. Chem Res Toxicol 27:1411–1420
Eick GN, Colucci JK, Harms MJ, Ortlund EA, Thornton JW (2012) Evolution of minimal specificity and promiscuity in steroid hormone receptors. PLoS Genet 8, e1003072
Ekuase EJ, Liu Y, Lehmler HJ, Robertson LW, Duffel MW (2011) Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1. Chem Res Toxicol 24:1720–1728
Ekuase EJ, Lehmler HJ, Robertson LW, Duffel MW (2014a) Binding interactions of hydroxylated polychlorinated biphenyls (OHPCBs) with human hydroxysteroid sulfotransferase hSULT2A1. Chem Biol Interact 212:56–64
Ekuase EJ, Lehmler HJ, Robertson LW, Duffel MW (2014) Binding interactions of hydroxylated polychlorinated biphenyls (OHPCBs) with human hydroxysteroid sulfotransferase hSULT2A1. Chem Biol Interact 212:56--64
Endo F, Monsees TK, Akaza H, Schill WB, Pflieger-Bruss S (2003) Effects of single non-ortho, mono-ortho, and di-ortho chlorinated biphenyls on cell functions and proliferation of the human prostatic carcinoma cell line, LNCaP. Reprod Toxicol 17:229–236
Erickson MD, Kaley RG 2nd (2011) Applications of polychlorinated biphenyls. Environ Sci Pollut Res Int 18:135–151
Fanini D, Palumbo G, Giorgi R, Pantaleoni G (1990) Behavioral effects of PCBs in mice. Behav Pharmacol 1:505–510
Goncharov A, Rej R, Negoita S, Schymura M, Santiago-Rivera A, Morse G, Akwesasne Task Force on the E, Carpenter DO (2009) Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men. Environ Health Perspect 117:1454–1460
Gray LE Jr, Kelce WR (1996) Latent effects of pesticides and toxic substances on sexual differentiation of rodents. Toxicol Ind Health 12:515–531
Gregoraszczuk EL, Rak A, Ludewig G, Gasinska A (2008) Effects of estradiol, PCB3, and their hydroxylated metabolites on proliferation, cell cycle, and apoptosis of human breast cancer cells. Environ Toxicol Pharmacol 25:227–233
Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW (2013) Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect 121:657–662
Grimm F, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW (2015) Metabolism and metabolites of polychlorinated biphenyls (PCBs). Crit Rev Toxicol 45:245--272
Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW (2015) Modulating inhibitors of transthyretin fibrillogenesis via sulfation: Polychlorinated biphenyl sulfates as models. Chem Biol Interact 228:1--8
Hedayatullah M, Leveque JC, Denivelle L (1972) Aryl and alkyl chlorosulfates and neutral sulfates. Action of sulfuryl chloride on alcohols. C R Seances Acad Sci D 274:1937–1940
Herrick RF, Meeker JD, Hauser R, Altshul L, Weymouth GA (2007) Serum PCB levels and congener profiles among US construction workers. Environ Heal 6:25
Hu D, Hornbuckle KC (2010) Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol 44:2822–2827
Hu D, Martinez A, Hornbuckle KC (2008) Discovery of non-aroclor PCB (3,3'-dichlorobiphenyl) in Chicago air. Environ Sci Technol 42:7873–7877
Jenkins JA, Olivier HM, Draugelis-Dale RO, Eilts BE, Torres L, Patino R, Nilsen E, Goodbred SL (2014) Assessing reproductive and endocrine parameters in male largescale suckers (Catostomus macrocheilus) along a contaminant gradient in the lower Columbia River, USA. Sci Total Environ 484:365–378
Joshi SN, Vyas SM, Duffel MW, Parkin S, Lehmler H-J (2011) Synthesis of sterically hindered polychlorinated biphenyl derivatives. Synthesis, 1045–1054
Kramer VJ, Helferich WG, Bergman A, Klasson-Wehler E, Giesy JP (1997) Hydroxylated polychlorinated biphenyl metabolites are anti-estrogenic in a stably transfected human breast adenocarcinoma (MCF7) cell line. Toxicol Appl Pharmacol 144:363–376
Krishnan V, Safe S (1993) Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) as antiestrogens in MCF-7 human breast cancer cells: quantitative structure-activity relationships. Toxicol Appl Pharmacol 120:55–61
Lathe R, Kotelevtsev Y (2014) Steroid signaling: ligand-binding promiscuity, molecular symmetry, and the need for gating. Steroids 82:14–22
Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Baan R, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working Group Iarc LF (2013) Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol 14:287–288
Lehmann GM, Christensen K, Maddaloni M, Phillips LJ (2014) Evaluating health risks from inhaled polychlorinated biphenyls: research needs for addressing uncertainty. Environ Health Perspect
Li X, Parkin S, Duffel MW, Robertson LW, Lehmler H-J (2010) An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ Int 36:843–848
Liu Y, Smart JT, Song Y, Lehmler HJ, Robertson LW, Duffel MW (2009) Structure-activity relationships for hydroxylated polychlorinated biphenyls as substrates and inhibitors of rat sulfotransferases and modification of these relationships by changes in thiol status. Drug Metab Dispos 37:1065–1072
Liu Y, Lehmler HJ, Robertson LW, Duffel MW (2011) Physicochemical properties of hydroxylated polychlorinated biphenyls aid in predicting their interactions with rat sulfotransferase 1A1 (rSULT1A1). Chem Biol Interact 189:153–160
Longnecker MP, Rogan WJ, Lucier G (1997) The human health effects of DDT (dichlorodiphenyltrichloroethane) and PCBS (polychlorinated biphenyls) and an overview of organochlorines in public health. Annu Rev Public Health 18:211–244
Ludewig G, Lehmann L, Esch H, Robertson LW (2008) Metabolic activation of PCBs to carcinogens in vivo - a review. Environ Toxicol Pharmacol 25:241–246
Machala M, Blaha L, Lehmler HJ, Pliskova M, Majkova Z, Kapplova P, Sovadinova I, Vondracek J, Malmberg T, Robertson LW (2004) Toxicity of hydroxylated and quinoid PCB metabolites: inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem Res Toxicol 17:340–347
Marek RF, Thorne PS, Wang K, Dewall J, Hornbuckle KC (2013) PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ Sci Technol 47:3353–3361
McLean MR, Bauer U, Amaro AR, Robertson LW (1996) Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol 9:158–164
National Research Council U.S (1999) Hormonally active agents in the environment. National Academy Press, Washington, 430 p.
Negri E, Bosetti C, Fattore E, La Vecchia C (2003) Environmental exposure to polychlorinated biphenyls (PCBs) and breast cancer: a systematic review of the epidemiological evidence. Eur J Cancer Prev 12:509–516
Oskam IC, Ropstad E, Dahl E, Lie E, Derocher AE, Wiig O, Larsen S, Wiger R, Skaare JU (2003) Organochlorines affect the major androgenic hormone, testosterone, in male polar bears (Ursus maritimus) at Svalbard. J Toxic Environ Health A 66:2119–2139
Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ljunggren J, Gustafsson JA, Carlquist M (1999) Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J 18:4608–4618
Pike AC, Brzozowski AM, Hubbard RE (2000) A structural biologist's view of the oestrogen receptor. J Steroid Biochem Mol Biol 74:261–268
Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC (2002) Polychlorinated biphenyls interfere with androgen-induced transcriptional activation and hormone binding. Toxicol Appl Pharmacol 179:185–194
Robertson LW, Hansen LG (2001) PCBs: recent advances in environmental toxicology and health effects. The University Press of Kentucky, Lexington
Ropstad E, Oskam IC, Lyche JL, Larsen HJ, Lie E, Haave M, Dahl E, Wiger R, Skaare JU (2006) Endocrine disruption induced by organochlorines (OCs): field studies and experimental models. J Toxic Environ Health A 69:53–76
Sacco JC, Lehmler HJ, Robertson LW, Li W, James MO (2008) Glucuronidation of polychlorinated biphenylols and UDP-glucuronic acid concentrations in channel catfish liver and intestine. Drug Metab Dispos 36:623–630
Safe S (1993) Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ Health Perspect 100:259–268
Safe SH (1994) Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24:87–149
Silberhorn EM, Glauert HP, Robertson LW (1990) Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol 20:440–496
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO (1995) The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect 103(Suppl 7):113–122
Soto AM, Michaelson CL, Prechtl NV, Weill BC, Sonnenschein C, Olea-Serrano F, Olea N (1998) Assays to measure estrogen and androgen agonists and antagonists. Adv Exp Med Biol 444:9–23, discussion p. 23--28
Sperry TS, Thomas P (1999) Identification of two nuclear androgen receptors in kelp bass (Paralabrax clathratus) and their binding affinities for xenobiotics: comparison with Atlantic croaker (Micropogonias undulatus) androgen receptors. Biol Reprod 61:1152–1161
Svobodova K, Plackova M, Novotna V, Cajthaml T (2009) Estrogenic and androgenic activity of PCBs, their chlorinated metabolites and other endocrine disruptors estimated with two in vitro yeast assays. Sci Total Environ 407:5921–5925
Szelei J, Jimenez J, Soto AM, Luizzi MF, Sonnenschein C (1997) Androgen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptor. Endocrinology 138:1406–1412
Tampal N, Lehmler HJ, Espandiari P, Malmberg T, Robertson LW (2002) Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs). Chem Res Toxicol 15:1259–1266
Telu S, Parkin S, Robertson LW, Lehmler H-J (2010) Improved syntheses of non-dioxin-like polychlorinated biphenyls (PCBs) and some of their sulfur-containing metabolites. Environ Int 36:828–834
Tue NM, Takahashi S, Suzuki G, Isobe T, Viet PH, Kobara Y, Seike N, Zhang G, Sudaryanto A, Tanabe S (2013) Contamination of indoor dust and air by polychlorinated biphenyls and brominated flame retardants and relevance of non-dietary exposure in Vietnamese informal e-waste recycling sites. Environ Int 51:160–167
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33:378–455
White R, Jobling S, Hoare SA, Sumpter JP, Parker MG (1994) Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 135:175–182
Zhai G, Lehmler H-J, Schnoor JL (2011) New hydroxylated metabolites of 4-monochlorobiphenyl in whole poplar plants. Chem Cent J 5:87
Zhai G, Lehmler HJ, Schnoor JL (2013) Sulfate metabolites of 4-monochlorobiphenyl in whole poplar plants. Environ Sci Technol 47:557–562
Zhang Q, Lu M, Wang C, Du J, Zhou P, Zhao M (2014) Characterization of estrogen receptor alpha activities in polychlorinated biphenyls by in vitro dual-luciferase reporter gene assay. Environ Pollut 189:169–175
Zoeller R, Vandenberg L (2015) Assessing dose–response relationships for endocrine disrupting chemicals (EDCs): a focus on non-monotonicity. Environ Health 14:42
Acknowledgments
We would like to thank Dr. Xueshu Li of the Synthesis Core of the Iowa Superfund Research Program for providing us with several PCB sulfates. This work was supported by NIEHS/NIH grant P42 ES013661 from the National Institute of Environmental Health Sciences, with programmatic support through the University of Iowa Environmental Health Sciences Research Center (NIEHS/NIH P30 ES05605).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 174 kb)
Rights and permissions
About this article
Cite this article
Flor, S., He, X., Lehmler, HJ. et al. Estrogenicity and androgenicity screening of PCB sulfate monoesters in human breast cancer MCF-7 cells. Environ Sci Pollut Res 23, 2186–2200 (2016). https://doi.org/10.1007/s11356-015-5142-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5142-y