Abstract
Purpose
To demonstrate that spectral analysis using the K114 fluorophore can detect and differentiate AL and AA renal amyloidosis.
Procedures
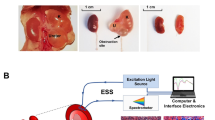
Kidney biopsies from patients with AL amyloidosis, AA amyloidosis, and normal samples with no evident pathology were stained with Congo Red and K114. The specimens were imaged on a spectral confocal microscope.
Results
Congo Red displayed homogeneous spectra across the three tissue types while K114 chromatically distinguished between normal tissue, AL amyloid, and AA amyloid. Additionally, Congo Red displayed an increased risk of false positive staining compared to K114. Spectral phasors computed from K114-stained tissue sections quantitatively differentiated the three tissue types. K114-stained amyloid deposits displayed a significantly greater increase in brightness after 50 images acquired in rapid succession compared to normal tissue. Quantitative analysis of intensity changes in the background of diseased tissue also differentiated AL and AA amyloid samples, suggesting widespread amyloid deposition. Both amyloid and the backgrounds of diseased samples red-shifted while normal tissue blue-shifted in response to repeated imaging, supporting this theory.
Conclusions
K114 staining of renal biopsies is a promising technique to detect and differentiate types of renal amyloidosis. Due to the advantages this method has over traditional Congo Red staining, the techniques presented here warrant further development for potential use in clinical settings.


Similar content being viewed by others
References
Herrera GA, Turbat-Herrera EA (2010) Renal diseases with organized deposits: an algorithmic approach to classification and clinicopathologic diagnosis. Arch Pathol Lab Med 134(4):512–531
Dember LM (2006) Amyloidosis-associated kidney disease. J Am Soc Nephrol 17(12):3458–3471
Khalighi MA, Dean Wallace W, Palma-Diaz MF (2014) Amyloid nephropathy. Clin Kidney J 7(2):97–106
Benson MD, Buxbaum JN, Eisenberg DS, Merlini G, Saraiva MJM, Sekijima Y, Sipe JD, Westermark P (2020) Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 27(4):217–222
Wu C, Scott J, Shea JE (2012) Binding of congo red to amyloid protofibrils of the Alzheimer Aβ(9–40) peptide probed by molecular dynamics simulations. Biophys J 103(3):550–557
Gupta N, Kaur H, Wajid S (2020) Renal amyloidosis: an update on diagnosis and pathogenesis. Protoplasma 257(5):1259
LeVine H 3rd (2005) Mechanism of Aβ(1–40) fibril-induced fluorescence of (trans, trans)-1-bromo-2,5-bis(4-hydroxystyryl)benzene (K114). Biochemistry 44(48):15937–15943
Stepanchuk AA, Heyne B, Stys PK (2021) Complex photophysical properties of K114 make for a versatile fluorescent probe for amyloid detection. ACS Chem Neurosci 12(7):1273–1280
Chu TH, Cummins K, Sparling JS, Tsutsui S, Brideau C, Nilsson KPR, Joseph JT, Stys PK (2017) Axonal and myelinic pathology in 5xFAD Alzheimer’s mouse spinal cord. PLoS One 12(11):e0188218
Stepanchuk AA, Joseph JT, Stys PK (2021) Spectral photokinetic conversion of the fluorescent probes BSB and K114 for improved detection of amyloid assemblies. J Biophotonics 14(12):e202100203
Stepanchuk AA, Barber PA, Lashley T, Joseph JT, Stys PK (2021) Quantitative detection of grey and white matter amyloid pathology using a combination of K114 and CRANAD-3 fluorescence. Neurobiol Dis 161:105540
Muruve DA, Mann MC, Chapman K, Wong JF, Ravani P, Page SA, Benediktsson H (2017) The biobank for the molecular classification of kidney disease: research translation and precision medicine in nephrology. BMC Nephrol 18(1):252
Alberta Precision Laboratories (2019) Ap07–2.03 alkaline congo red-puchters
Stys PK (2021) Imagetrak homepage https://stysneurolab.org/imagetrak/
Fereidouni F, Bader AN, Gerritsen HC (2012) Spectral phasor analysis allows rapid and reliable unmixing of fluorescence microscopy spectral images. Opt Express 20(12):12729–12741
Golfetto O, Hinde E, Gratton E (2015) The laurdan spectral phasor method to explore membrane micro-heterogeneity and lipid domains in live cells. Methods Mol Biol 1232:273–290
Stepanchuk AA, Tahir W, Nilsson KPR, Schatzl HM, Stys PK (2021) Early detection of prion protein aggregation with a fluorescent pentameric oligothiophene probe using spectral confocal microscopy. J Neurochem 156(6):1033–1048
Acknowledgements
We thank Craig Brideau for providing technical support and Kevin Chapman for assisting in the preparation of the biopsy specimens.
Funding
Funding for the study was provided by the Department of Medicine, University of Calgary, an Alberta Prion Research Institute Explorations grant, and a Krembil Foundation grant.
Author information
Authors and Affiliations
Contributions
PJB performed the experiments and contributed to data analysis. AAS assisted with the experimental design and data analysis. GA assisted with literature review, study design, and methods. PKS wrote the image analysis software and contributed to study design and data analysis. PKS and DAM conceived of the work. DAM assisted with study design, interpreting results, and wrote the first draft of the manuscript. All authors contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
PKS holds an issued patent on methods to detect diseases with abnormal protein aggregation. The other authors declare no other conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brandt, P.J., Stepanchuk, A.A., Andonegui, G. et al. Detection and Typing of Renal Amyloidosis by Fluorescence Spectroscopy Using the Environmentally Sensitive Fluorophore K114. Mol Imaging Biol 25, 221–227 (2023). https://doi.org/10.1007/s11307-022-01754-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01754-w