Abstract
Purpose
LRRK2 (leucine-rich repeat kinase 2) has recently been proven to be a promising drug target for Parkinson’s disease (PD) due to an apparent enhanced activity caused by mutations associated with familial PD. To date, there have been no reports in which a LRRK2 inhibitor has been radiolabeled and used for in in vitro or in vivo studies of LRRK2. In the present study, we radiolabeled the LRRK2 ligand, LRRK-IN-1, for the purposes of performing in vitro (IC50, K d , B max, autoradiography) and in vivo (biodistribution, and blocking experiments) evaluations in rodents and human striatum tissues.
Procedures
[3H]LRRK2-IN-1 was prepared with high radiochemical purity (>99 %) and a specific activity of 41 Ci/mmol via tritium/hydrogen (T/H) exchange using Crabtree’s catalyst. For IC50, K d , and B max determination, LRRK2-IN-1 was used as a competing drug for nonspecific binding assessment. The specific binding of the tracer was further evaluated via an in vivo blocking study in mice with a potent LRRK2 inhibitor, Pf-06447475.
Results
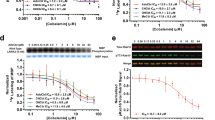
In vitro binding studies demonstrated a saturable binding site for [3H]LRRK2-IN-1 in rat kidney, rat brain striatum and human brain striatum with K d of 26 ± 3 and 43 ± 8, 48 ± 2 nM, respectively. In rat, the density of LRRK2 binding sites (B max) was higher in kidney (6.4 ± 0.04 pmol/mg) than in brain (2.5 ± 0.03 pmol/mg), however, in human brain striatum, the B max was 0.73 ± 0.01 pmol/mg protein. Autoradiography imaging in striatum of rat and human brain tissues gave results consistent with binding studies. In in vivo biodistribution and blocking studies in mice, co-administration with Pf-06447475 (10 mg/kg) reduced the uptake of [3H]LRRK2-IN-1 (%ID/g) by 50–60% in the kidney or brain.
Conclusion
The high LRRK2 brain density observed in our study suggests the feasibility for positron emission tomography imaging of LRRK2 (a potential target) with radioligands of higher affinity and specificity.






Similar content being viewed by others
Change history
01 May 2017
An erratum to this article has been published.
References
Bosgraaf L, van Haastert PJ (2003) Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta 1643:5–10
Gilsbach BK, Kortholt A (2014) Structural biology of the LRRK2 GTPase and kinase domains: implications for regulation. Front Mol Neurosci 7:32
West AB, Moore DJ, Biskup S et al (2005) Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A 102:16842–16847
Smith WW, Pei Z, Jiang H et al (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9:1231–1233
Bonifati V (2006) The LRRK2-G2019S mutation: opening a novel era in Parkinson’s disease genetics. Eur J Hum Genet 14:1061–1062
Luzón-Toro B, Rubio de la Torre E, Delgado A (2007) Mechanistic insight into the dominant mode of the Parkinson’s disease-associated G2019S LRRK2 mutation. Hum Mol Genet 16:2031–2039
Jaleel M, Nichols RJ, Deak M et al (2007) LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J 405:307–317
Anand VS, Reichling LJ, Lipinski K et al (2009) Investigation of leucine-rich repeat kinase 2: enzymological properties and novel assays. FEBS J 276:466–478
Paisan-Ruiz C, Jain S, Evans EW et al (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44:595–600
Zimprich A, Biskup S, Leitner P et al (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–607
Adams JR, van Netten H, Schulzer M et al (2005) PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain 128(Pt 12):2777–2785
Guo L, Gandhi PN, Wang W et al (2007) The Parkinson’s disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res 313:3658–3670
Cookson MR, Bandmann O (2010) Parkinson’s disease: insights from pathways. Hum Mol Genet 19:R21–R27
Cookson MR (2010) The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci 11:791–797
Daechsel JC, Farrer MJ (2010) LRRK2 and Parkinson disease. Arch Neurol 67:542–547
Lewis PA, Greggio E, Beilina A et al (2007) The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun 357:668–671
Sen S, Webber PJ, West AB (2009) Dependence of leucine-rich repeat kinase 2 (LRRK2) kinase activity on dimerization. J Biol Chem 284:36346–36356
Berger Z, Smith KA, LaVoie MJ (2010) Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemist 49:5511–5523
Nichols RJ, Dzamko N, Morrice NA et al (2010) 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem J 430:393–404
Civiero L, Vancraenenbroeck R, Belluzzi E et al (2012) Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers. PLoS One 7:e43472
James NG, Digman MA, Gratton E et al (2012) Number and brightness analysis of LRRK2 oligomerization in live cells. Biophys J 102:L41–L43
Bahnassawy L, Nicklas S, Palm T et al (2013) The Parkinson’s disease-associated LRRK2 mutation R1441G inhibits neuronal differentiation of neural stem cells. Stem Cells Dev 22:2487–2496
Zhang J, Yang PL, Gray NS (2009) Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9:28–39
Deng X, Dzamko N, Prescott A et al (2011) Characterization of a selective inhibitor of the Parkinson’s disease kinase LRRK2. Nat Chem Biol 7:203–205
Reith AD, Bamborough P, Jandu K et al (2012) GSK2578215A; a potent and highly selective 2-arylmethyloxy-5-substitutent-N-arylbenzamide LRRK2 kinase inhibitor. Bioorg Med Chem Lett 22:5625–5629
Estrada AA, Liu X, Baker-Glenn C et al (2012) Discovery of highly potent, selective, and brain-penetrable leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J Med Chem 55:9416–9433
Kavanagh ME, Doddareddy MR, Kassiou M (2013) The development of CNS-active LRRK2 inhibitors using property-directed optimisation. Bioorg Med Chem Lett 23:3690–3696
Davies P, Hinkle KM, Sukar NN et al (2013) Comprehensive characterization and optimization of anti-LRRK2 (leucine-rich repeat kinase 2) monoclonal antibodies. Biochem J 453:101–113
Estrada AA, Chan BK, Baker-Glenn C et al (2014) Discovery of highly potent, selective, and brain-penetrant aminopyrazole leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J Med Chem 57:921–936
Henderson JL, Kormos BL, Hayward MM et al (2015) Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7H-pyrrolo[2, 3-d]pyrimidin-5-yl] benzonitrile (Pf-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor. J Med Chem 58:419–432
Daher JP, Abdelmotilib HA, Hu X et al (2015) Leucine-rich repeat kinase 2 (LRRK2) pharmacological inhibition abates α-synuclein gene-induced neurodegeneration. J Biol Chem 290:19433–19444
Koshibu K, van Asperen J, Gerets H et al (2015) Alternative to LRRK2-IN-1 for pharmacological studies of Parkinson’s disease. Pharmacology 96:240–247
Liu Z, Galemmo RA Jr, Fraser KB et al (2014) Unique functional and structural properties of the LRRK2 protein ATP-binding pocket. J Biol Chem 289:32937–32951
Gray NS, Waller D, Choi HG et al (2014) Pyrimido-diazepinone compounds and methods of treating disorders. Patent: WO 2014145909:A3
Dzamko N, Deak M, Hentati F et al (2010) Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem J 430:405–413
Taylor SS, Kornev AP (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci 36:65–77
Giesert F, Hofmann A, Buerger A et al (2013) Expression analysis of Lrrk1, Lrrk2 and Lrrk2 splice variants in mice. PLoS One 8:e63778
West AB, Cowell RM, Daher JP et al (2014) Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J Comp Neurol 522:2465–2480
Dorval V, Mandemakers W, Jolivette F et al (2014) Gene and MicroRNA transcriptome analysis of Parkinson’s related LRRK2 mouse models. PLoS One 9:e85510
Maekawa T, Kubo M, Yokoyama I et al (2010) Age-dependent and cell-population-restricted LRRK2 expression in normal mouse spleen. Biochem Biophys Res Commun 392:431–435
Taymans JM, Van den Haute C, Baekelandt V (2006) Distribution of PINK1 and LRRK2 in rat and mouse brain. J Neurochem 98:951–961
Miklossy J, Arai T, Guo JP et al (2006) LRRK2 expression in normal and pathologic human brain and in human cell lines. J Neuropathol Exp Neurol 65:953–963
Westerlund M, Belin AC, Anvret A et al (2008) Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: implications for Parkinson’s disease. Neuroscience 152:429–436
Herzig MC, Kolly C, Persohn E et al (2010) LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet 20:4209–4223
Baptista MA, Dave KD, Frasier MA et al (2012) Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS One 8:e80705
Tong Y, Giaime E, Yamaguchi H et al (2012) Loss of leucine-rich repeat kinase 2 causes age-dependent bi-phasic alterations of the autophagy pathway. Mol Neurodegener 7:2
Fell MJ, Mirescu C, Basu K et al (2015) MLi-2, a potent, selective, and centrally active compound for exploring the therapeutic potential and safety of LRRK2 kinase inhibition. J Pharmacol Exp Ther 355:397–409
Joyce JN, Sapp DW, Marshall JF (1986) Human striatal dopamine receptors are organized in compartments. Proc Nat Acad Sci USA 83:8002–8006
Hall H, Sedvall G, Magnusson O et al (1994) Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacol 11:245–256
Quirion R, Pilapil C, Allaoua H et al (1995) Autoradiographic distribution of multiple opioid, sigma and phencyclidine receptor binding sites in the human brain. In: Biegon A, Volkow ND (eds) ISBN: 084937653XSites of drug action in the human brain, pp 117–141
Kulkarni AD, Patel HM, Surana SJ et al (2016) Brain-blood ratio: implications in brain drug delivery. Expert Opin Drug Deliv 13:85–92
Convents A, De Keyser J, De Backer JP et al (1989) [3H] rauwolscine labels alpha 2-adrenoceptors and 5-HT1A receptors in human cerebral cortex. Eur J Pharmacol 159(3):307–310
Durany N, Zoechling R, Boissl KW et al (2000) Human post-mortem striatal α4β2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson's syndrome. Neurosci Lett 287:109–112
Traut TW (1994) Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 140:1–22
Thermofisher Tools and protocols on kinase basic module—theory: https://tools.thermofisher.com/content/sfs/manuals/LRRK2_G2019S_LanthaScreen_Activity.pdf
Hicks JW, VanBrocklin HF, Wilson AA et al (2010) Radiolabeled small molecule protein kinase inhibitors for imaging with PET or SPECT. Molecules 15:8260–8278
Sun J, Cai L, Zhang K et al (2014) A pilot study on EGFR-targeted molecular imaging of PET/CT with 11C-PD153035 in human gliomas. Clin Nucl Med 39:e20–e26
Acknowledgements
The work is supported by the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) at New York University School of Medicine is supported by NIH/NIBIB grant number P41 EB017183. We would like to acknowledge the expert assistance of Mr. Yianni Piyis (METIS Laboratories) for help with the sectioning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11307-017-1086-6.
Rights and permissions
About this article
Cite this article
Malik, N., Gifford, A.N., Sandell, J. et al. Synthesis and In Vitro and In Vivo Evaluation of [3H]LRRK2-IN-1 as a Novel Radioligand for LRRK2. Mol Imaging Biol 19, 837–845 (2017). https://doi.org/10.1007/s11307-017-1070-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-017-1070-1