Abstract
Purpose
The goal of this study was to improve the pharmacokinetic properties and specificity of an ERBB2-targeted peptide for SPECT imaging.
Procedures
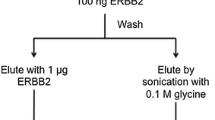
Bacteriophages (phages) displaying the ERBB2 targeting sequence, KCCYSL, flanked by additional random amino acids were used for in vivo selections in mice-bearing ERBB2-expressing MDA-MB-435 human breast xenografts. Phage-displayed peptides were evaluated for ERBB2 and cancer cell binding affinity and specificity in vitro, and one peptide was radiolabeled with 111In-DOTA and biodistribution and SPECT imaging properties were compared to the first generation peptide, 111In-DOTA-KCCYSL.
Results
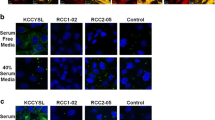
In vivo phage display selected two peptides, 1-D03 (MEGPSKCCYSLALSH) and 3-G03 (SGTKSKCCYSLRRSS), with higher breast carcinoma cell specificity and similar ErbB2 affinity (236 and 289 nM, respectively) to the first generation peptide. The corresponding radiolabeled probes bound with higher affinity to target cancer cells than 111In-DOTA-KCCYSL; however, only 111In-DOTA-1-D03 demonstrated higher specificity for MDA-MB-435 cells. Biodistribution analysis demonstrated that although 111In-DOTA-1-D03 had slightly reduced tumor uptake (0.661 % ID/g) in comparison to 111In-DOTA-KCCYSL (0.78 %/ID/g), its dramatic improvement in blood clearance led to a significantly higher tumor/blood ratio (6.02:1). Non-specific uptake was also reduced in most organs including heart, lung, muscle, bone, and kidneys. SPECT imaging revealed tumor-specific uptake of 111In-DOTA-1-D03, which was confirmed by blocking with unlabeled 1-D03 peptide.
Conclusions
This is the first evidence that SPECT imaging peptides with improved tumor specificity and pharmacokinetics can be obtained by in vivo phage display affinity maturation. The combination of ERBB2-specific binding, rapid clearance, and tumor specificity may make 1-D03 a viable candidate for clinical imaging studies.






Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Alroy I (1997) The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand–receptor interactions. FEBS Lett 410:83
Graus-Porta D, Beerli RR, Daly JM, Hynes NE (1997) ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16:1647–1655
Hynes NE, Stern DF (1994) The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta 1198:165–184
Witton CJ, Reeves JR, Going JJ et al (2003) Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol 200:290–297
Milenic DE, Yokota T, Filpula DR et al (1991) Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res 51:6363–6371
Fujimori K (1990) A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med 31:1191
Landon LA, Zou J, Deutscher SL (2004) Is phage display technology on target for developing peptide-based cancer drugs? Curr Drug Dis Technol 1:113–132
Landon LA, Deutscher SL (2003) Combinatorial discovery of tumor targeting peptides using phage display. J Cell Biochem 90:509–517
Peletskaya E, Glinsky G, Deutscher S, Quinn T (1996) Identification of peptide sequences that bind the Thomsen-Friedenreich cancer-associated glycoantigen from bacteriophage peptide display libraries. Mol Divers 2:13–18
Ruoslahti E (2000) Targeting tumor vasculature with homing peptides from phage display. Semin Cancer Biol 10:435–442
Karasseva NG, Glinsky VV, Chen NX et al (2002) Identification and characterization of peptides that bind human ErbB-2 selected from a bacteriophage display library. J Protein Chem 21:287–296
Kumar SR, Quinn TP, Deutscher SL (2007) Evaluation of an 111In-radiolabeled peptide as a targeting and imaging agent for ErbB-2 receptor-expressing breast carcinomas. Clin Cancer Res 13:6070–6079
Deutscher SL, Figueroa SD, Kumar SR (2009) 111In-labeled KCCYSL peptide as an imaging probe for ErbB-2-expressing ovarian carcinomas. J Label Compd Radiopharm 52:583–590
Kocks C, Rajewsky K (1988) Stepwise intraclonal maturation of antibody affinity through somatic hypermutation. Proc Natl Acad Sci U S A 85:8206–8210
Landon LA, Peletskaya EN, Glinsky VV et al (2003) Combinatorial evolution of high-affinity peptides that bind to the Thomsen-Friedenreich carcinoma antigen. J Protein Chem 22:193–204
Smith GP G.P. Smith Phage Display Website. http://www.biosci.missouri.edu/smithGP/PhageDisplayWebsite/PhageDisplayWebsiteIndex.html
Newton JR, Kelly KA, Mahmood U et al (2006) In vivo selection of phage for the optical imaging of PC-3 human prostate carcinoma in mice. Neoplasia 8:772–780
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Huang J, Ru B, Li S, Lin H, Guo F-B (2010) SAROTUP: scanner and reporter of target-unrelated peptides. J Biomed Biotechnol. doi:10.1155/2010/101932
Franklin MC, Carey KD, Vajdos FF et al (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5:317–328
Cho H-S, Mason K, Ramyar KX et al (2003) Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421:756–760
Molina MA, Codony-Servat J, Albanell J et al (2001) Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res 61:4744–4749
Lobo ED, Hansen RJ, Balthasar JP (2004) Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci 93:2645–2668
Zhang X, Cabral P, Bates M et al (2010) In vitro and in vivo evaluation of [99mTc (CO) 3]-radiolabeled ErbB-2-targeting peptides for breast carcinoma imaging. Curr Radiopharm 3:308–321
Vegt E, de Jong M, Wetzels JFM et al (2010) Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med 51:1049–1058
Miao Y, Fisher DR, Quinn TP (2006) Reducing renal uptake of 90Y- and 177Lu-labeled alpha-melanocyte stimulating hormone peptide analogues. Nucl Med Biol 33:723–733
Hoffman TJ, Gali H, Smith CJ et al (2003) Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med 44:823–831
Froidevaux S, Eberle AN, Christe M et al (2002) Neuroendocrine tumor targeting: study of novel gallium-labeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer 98:930–937
Good S, Walter MA, Waser B et al (2008) Macrocyclic chelator-coupled gastrin-based radiopharmaceuticals for targeting of gastrin receptor-expressing tumours. Eur J Nucl Med 35:1868–1877
Chen J, Cheng Z, Owen NK et al (2001) Evaluation of an 111In-DOTA–rhenium cyclized α-MSH analog: a novel cyclic-peptide analog with improved tumor-targeting properties. J Nucl Med 42:1847–1855
Deutscher SL (2010) Phage display in molecular imaging and diagnosis of cancer. Chem Rev 110:3196–3211
Derda R, Tang S, Li SC, Ng S et al (2011) Diversity of phage-displayed libraries of peptides during panning and amplification. Molecules 16:1776–1803
Olsen JO, Pozderac RV, Hinkle G et al (1995) Somatostatin receptor imaging of neuroendocrine tumors with indium-111 pentetreotide (Octreoscan). Semin Nucl Med 25:251–261
Liu S, Edwards DS (1999) 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev 99:2235–2268
Yu D, Hung M-C (2000) Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 19:6115
Krenning EP, Kwekkeboom DJ, Bakker WH et al (1993) Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 20:716–731
Béhé M, Becker W, Gotthardt M et al (2003) Improved kinetic stability of DTPA-dGlu as compared with conventional monofunctional DTPA in chelating indium and yttrium: preclinical and initial clinical evaluation of radiometal labelled minigastrin derivatives. Eur J Nucl Med Mol Imaging 30:1140–1146
Ferro-Flores G, de Murphy CA, Rodr`guez-Corte`s J et al (2006) Preparation and evaluation of 99mTc-EDDA/HYNIC-[Lys3]-bombesin for imaging gastrin-releasing peptide receptor-positive tumours. Nucl Med Commun 27:371–376
Thakur ML, Aruva MR, Gariepy J et al (2004) PET imaging of oncogene overexpression using 64Cu-vasoactive intestinal peptide (VIP) analog: comparison with 99mTc-VIP analog. J Nucl Med 45:1381–1389
Rolleman E, Valkema R, de Jong M et al (2003) Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging 30:9–15
Vegt E, van Eerd JEM, Eek A et al (2008) Reducing renal uptake of radiolabeled peptides using albumin fragments. J Nucl Med 49:1506–1511
Acknowledgments
The authors would like to acknowledge the contributions of Jessica Newton-Northup, Marie T. Dickerson, and the VA Biomolecular Imaging Core. Work funded by Department of Veterans Affairs VA Merit 5I0 1BX000964.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larimer, B.M., Thomas, W.D., Smith, G.P. et al. Affinity Maturation of an ERBB2-Targeted SPECT Imaging Peptide by In Vivo Phage Display. Mol Imaging Biol 16, 449–458 (2014). https://doi.org/10.1007/s11307-014-0724-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-014-0724-5