Abstract
Purpose
The aim of the present work was to perform the labelling of granulocytes by their engulfment with chitosan-coated magnetic 64Cu nanoparticles (MNPs) in order to obtain a radiopharmaceutical suitable for dual imaging (PET–MRI) of inflammatory/infective diseases.
Procedures
Specimens of 5–20 mg MNPs were washed with saline–isotonic solution and recuperated by magnetic decantation; 15–58 μg Cu2+ (CuCl2·H2O) in 50 μl of acidified (pH 5.5) saline solution was added to the MNPs re-suspended saline–isotonic solution; 10 mg MNPs was allowed to react with 16 μg 64Cu [64Ni(p,n) at 12–9 MeV] followed by anion exchange chromatography with a specific activity of 56 MBq/μg. Pellets of granulocytes were obtained from peripheral blood; MNPs engulfment by granulocytes was obtained and granulocyte-engulfed viability was assessed by the trypan blue exclusion (TBE) test performed at 5 min, 2 h and 4 h; assessment of the release of 64Cu from labelled granulocytes in plasma was performed by measuring the radioactivity of both the cellular pellet and the supernatant solution.
Results
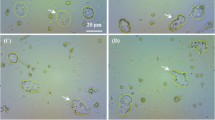
Our data showed the binding capacity of chitosan-coated MNPs for cationic metal. The amount of Cu+2 chelated captured per milligram of MNPs was constant and independent of the reagent concentrations. In all cases, more than 90% of the engulfed granulocytes were positive to the TBE test. The MNPs were localised within the cells.
Conclusion
In our in vitro model, MNPs are taken up by granulocytes through phagocytosis, whereas previously described methods were based on the use of a chelating agent that permit Cu to cross the cell membrane. Moreover, the 64Cu-engulfed granulocytes showed a high stability of up to 80% of retained radioactivity after 24 h of incubation.


Similar content being viewed by others
References
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49
Wang Y-XJ, Hussain SM, Krestin GP (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 11:2319–2331
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–4021
Gupta AK, Naregalkar RR, Vaidya VD, Gupta M (2007) Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2:23–39
Laurent S, Forge D, Port M et al (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110
Montet X, Montet-Abou K, Reynolds F et al (2006) Nanoparticle imaging of integrins on tumor cells. Neoplasia 8:214–222
Nahrendorf M, Zhang H, Hembrador S et al (2008) Nanoparticle PET–CT imaging of macrophages in inflammatory atherosclerosis. Circulation 117:379–387
Choi JS, Park JC, Nah H et al (2008) A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed Engl 47:6259–6262
Xu C, Xie J, Ho D et al (2008) Au–Fe3O4 dumbbell nanoparticles as dual-functional probes. Angew Chem Int Ed Engl 47:173–176
Lee S, Chen X (2009) Dual-modality probes for in vivo molecular imaging. Mol Imaging 8:87–100
Lee HY, Li Z, Chen et al (2008) PET/MRI dual-modality tumor imaging using arginine–glycine–aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med 49:1371–1379
Lee JH, Lee K, Moon SH et al (2009) All-in-one target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew Chem Int Ed Engl 48:4174–4179
Weisseleder R (2002) Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer 2:11–18
Cheon J, Lee JH (2008) Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc Chem Res 41:1630–1640
Jones HA, Peters AM, Clark JC (2001) 18F-FDG labelling of human leukocytes. Nucl Med Commun 22:601–602
Forstrom LA, Mullan BP, Hung JC et al (2000) 18F-FDG labelling of human leukocytes. Nucl Med Commun 21:691–694
Basu S, Zhuang H, Torigian DA et al (2009) Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med 39:124–145
Zhuang H, Yu JQ, Alavi A (2005) Applications of fluorodeoxyglucose–PET imaging in the detection of infection and inflammation and other benign disorders. Radiol Clin North Am 43:121–134
Reni JN, Bhargava KK, Tronco GG et al (2006) PET with FDG-labeled leukocytes versus scintigraphy with 111In-oxine-labeled leukocytes for detection of infection. Radiology 238:978–987
Dumarey ND, Egrise D, Blocklet B et al (2006) Imaging infection with 18F-FDG-labeled leukocyte PET/CT: initial experience in 21 patients. J Nucl Med 47:625–632
Rini JN, Palestro CJ (2006) Imaging of infection and inflammation with 18F-FDG-labeled leukocytes. Q J Nucl Med Mol Imaging 50:143–146
Pellegrino D, Bonab AA, Dragotakes SC et al (2005) Inflammation and infection: imaging properties of 18F-FDG-labeled white blood cells versus 18F-FDG. J Nucl Med 46:1522–1530
Bhargava KK, Gupta RK, Nichols KJ, Palestro CJ (2009) In vitro human leukocyte labelling with 64Cu: an intraindividual comparison with 111In-oxine and 18F-FDG. Nucl Med Biol 36:545–549
Wang YXJ, Wang HH, Au DWT et al (2008) Pitfalls in employing superparamagnetic iron oxide particles for stem cell labelling and in vivo MRI tracking. Br J Radiol 81:987–988
Tsopelas C (2005) The radiopharmaceutical chemistry of 99mTc–tin fluoride colloid-labeled-leukocytes. Q J Nucl Med Mol Imaging 49:319–324
Capriotti G, D’Alessandria C, Garin E et al (2004) An in vitro study to compare 99mTc-stannous colloids and 99mTc-HMPAO for labelling human leukocytes. Q J Nucl Med 48:229–236
Mowat P, Franconi F, Chapon C et al (2007) Evaluating SPIO-labelled cell MR efficiency by three-dimensional quantitative T2* MRI. NMR Biomed 20:21–27
Montet-Abou K, Daire JL, Hyacinthe JN et al (2010) In vivo labelling of resting monocytes in the reticuloendothelial system with fluorescent iron oxide nanoparticles prior to injury reveals that they are mobilized to infarcted myocardium. Eur Heart J 31:1410–1420
Garden OA, Reynolds PR, Yates J et al (2006) A rapid method for labelling CD4+ T cells with ultrasmall paramagnetic iron oxide nanoparticles for magnetic resonance imaging that preserves proliferative, regulatory and migratory behaviour in vitro. J Immunol Methods 314:123–133
Singh RP, Kumar S, Nada R, Prasad R (2006) Evaluation of copper toxicity in isolated human peripheral blood mononuclear cells and its attenuation by zinc: ex vivo. Mol Cell Biochem 282:13–21
Acknowledgement
The authors are deeply indebted to the Italian Company ACOM, Montecosaro, Macerata (Italy), for providing us 64Cu and for having made available equipment and expertise for the use of the radioisotope.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pala, A., Liberatore, M., D’Elia, P. et al. Labelling of Granulocytes by Phagocytic Engulfment with 64Cu-Labelled Chitosan-Coated Magnetic Nanoparticles. Mol Imaging Biol 14, 593–598 (2012). https://doi.org/10.1007/s11307-011-0526-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0526-y