Abstract
Purpose
Animal models are important for pre-clinical assessment of novel therapies in metastatic bladder cancer. The F344/AY-27 model involves orthotopic colonisation with AY-27 tumour cells which are syngeneic to F344 rats. One disadvantage of the model is the unknown status of colonisation between instillation and sacrifice. Non-invasive optical imaging using red fluorescence reporters could potentially detect tumours in situ and would also reduce the number of animals required for each experiment.
Materials and Methods
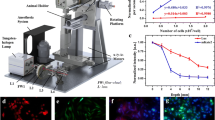
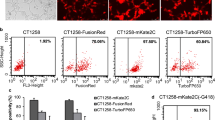
AY-27 cells were stably transfected with either pDsRed2-N1 or pcDNA3.1tdTomato. The intensity and stability of fluorescence in the resultant AY-27/DsRed2-N1 and AY-27/tdTomato stable cell lines were compared using Xenogen IVIS®200 and Olympus IX51 systems.
Results
AY-27/tdTomato fluorescence intensity was 60-fold brighter than AY27/DsRed2-N1 and was sustained in AY-27/tdTomato cells following freezing and six subsequent sub-cultures. After sub-cutaneous injection, fluorescence intensity from AY-27/tdTomato cells was threefold stronger than that detected from AY-27/DsRed2-N1 cells. IVIS®200 detected fluorescence from AY-27/tdTomato and AY-27/DsRed2-N1 cells colonising resected and exteriorised bladders, respectively. However, the deep-seated position of the bladder precluded in vivo imaging. Characteristics of AY-27/tdTomato cells in vitro and in tumours colonising F344 rats resembled those of parental AY-27 cells. Tumour transformation was observed in the bladders colonised with AY-27/DsRed2-N1 cells.
Conclusions
In vivo whole-body imaging of internal red fluorescent animal tumours should use pcDNA3.1tdTomato rather than pDsRed2-N1. Optical imaging of deep-seated organs in larger animals remains a challenge which may require proteins with brighter red or far-red fluorescence and/or alternative approaches.







Similar content being viewed by others
References
Xiao Z, McCallum TJ, Brown KM et al (1999) Characterization of a novel transplantable orthotopic rat bladder transitional cell tumor model. Br J Cancer 81:638–646
Asanuma H, Arai T, Seguchi K et al (2003) Successful diagnosis of orthotopic rat superficial bladder tumor by ultrathin cystoscopy. J Urol 169:718–720
Yang M, Jiang P, Yamamoto N et al (2005) Real-time whole-body imaging of an orthotopic metastatic prostate cancer model expressing red fluorescent protein. Prostate 62:374–379
Winnard PT, Kluth JB, Raman V (2006) Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia 8:796–806
Gross LA, Baird GS, Hoffman RC et al (2000) The structure of the chromophore within DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci USA 97:11990–11995
Shaner NC, Steinbach PA, Tsien RY (2005) A guide to choosing fluorescent proteins. Nat Methods 2:905–909
Zhou J-H, Rosser CJ, Tanaka M et al (2002) Visualizing superficial human bladder cancer cell growth in vivo by green fluorescent protein expression. Cancer Gene Therapy 9:681–686
Yang M, Luiken G, Baranov E, Hoffman RM (2005) Biotechniques 39:170–172
Hoffman RM (2005) The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer 5:796–806
Hoffman RM, Yang M (2006) Whole-body imaging with fluorescent proteins. Nature Protocols 1:1429–1438
Weber J, Weberova J, Carobene M et al (2006) Use of a novel assay based on intact recombinant viruses expressing green (EGFP) or red (DsRed2) fluorescent proteins to examine the contribution of pol and env genes to overall HIV-1 replicative fitness. J Virol Methods 136:102–117
Seitz G, Warmann SW, Fuchs J et al (2006) Visualization of xenotransplanted human rhabdomyosarcoma after transfection with red fluorescent protein. J Pediatr Surg 41:1369–1376
Hadjantonakis AK, Papaioannou VE (2004) Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol 4:33
Maruyama M, Nishio T, Yoshida T et al (2004) Simultaneous detection of DsRed2-tagged and EGFP-tagged human beta-interferons in the same single cells. J Cell Biochem 93:497–502
Harris AL, Neal DE (1992) Bladder cancer—field versus clonal origin. N Engl J Med 326:759–769
Campbell RE, Tour O, Palmer AE et al (2002) A monomeric red fluorescent protein. Proc Natl Acad Sci USA 99:7877–7882
Bevis BJ, Glick BS (2002) Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat Biotechnol 20:83–87
Scherbo D, Merzlyak EM, Chepurnykh TV et al (2007) Bright far-red fluorescent protein for whole-body imaging. Nat Methods 4:741–746
Acknowledgements
The authors thank Professor Roger Tsien (University California of San Diego, USA) for the kind gift of the tdTomato gene in pRSET-B vector. This project has been funded by Research and Development Offices, HPSS Northern Ireland (AL); Mason’s Medical Foundation (VK); Egyptian Cultural Bureau (OS); Department of Employment and Learning (CW). We would like to thank Mr. Ken Arthur for his help with DNA flow cytometry.
Ethical Approval
All animal studies described in this manuscript were conducted according to Animal Licencing guidelines and approval.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koo, V., Lee, A., Sharaf Eldin, O. et al. pcDNA3.1tdTomato Is Superior to pDsRed2-N1 for Optical Fluorescence Imaging in the F344/AY-27 Rat Model of Bladder Cancer. Mol Imaging Biol 12, 509–519 (2010). https://doi.org/10.1007/s11307-009-0275-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0275-3