Abstract
Introduction
Untargeted metabolomics of cord blood indicated that antiretroviral therapy to HIV-infected mothers (HIV-ART) did not compromise the exposed neonates with regard to the stress of neonatal hypoglycaemia at birth. However, identified biomarkers reflected stress in their energy metabolism, raising concern over developmental risks in some newborns exposed to ART.
Objectives
This study addresses the concern over HIV-ART-induced metabolic perturbations by expanding the metabolomics study to the amino acid profiles in cord blood collected at birth from newborns either exposed or unexposed to HIV-ART in utero.
Methods
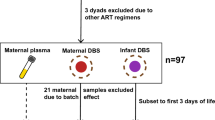
Amino acid profiles derived from liquid chromatographic triple quadruple spectra of cord blood from neonates exposed and unexposed to HIV-ART (cohort 1) were investigated using a metabolomics approach. Amino acid data, generated by ultra performance liquid chromatography–tandem mass spectrometry from similar cases (cohort 2), were included for comparison.
Results
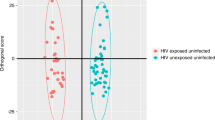
Multivariate and supporting statistics indicated differentiation between the exposed and unexposed neonates in both cohorts, caused by a general decrease or downregulation of amino acid concentrations in the cord blood samples from the exposed cases. Specifically, significant upregulation of aspartic acid in both cohorts and downregulation of arginine, and of threonine, tryptophan and lysine in cohorts 1 and 2, respectively, were observed.
Conclusions
The benefits of ART for HIV-infected pregnant women are well established. However, the amino acid profile of cord blood, obtained from the two independent cohorts, adds to observed metabolic risks of in utero HIV-ART-exposed newborns. These risks could potentially have adverse consequences for the future health of some exposed infants.




Similar content being viewed by others
References
Barker, D. J. (1998). In utero programming of chronic disease. Clinical Science, 95(2), 115–128.
Barret, B., Tardieu, M., Rustin, P., Lacroix, C., Chabrol, B., Desguerre, I., et al. (2003). Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS (London, England), 17(12), 1769–1785.
Blanche, S., Tardieu, M., Rustin, P., Slama, A., Barret, B., Firtion, G., et al. (1999). Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet, 354, 1084–1089.
Brogly, S. B., Ylitalo, N., Mofenson, L. M., Oleske, J., Van Dyke, R., Crain, M. J., et al. (2007). In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS (London, England), 21(8), 929–938.
Cassol, E., Misra, V., Morgello, S., Kirk, G. D., Mehta, S. H., & Gabuzda, D. (2015). Altered monoamine and acylcarnitine metabolites in HIV-positive and HIV-negative subjects with depression. Journal of Acquired Immune Deficiency Syndrome, 69(1), 18–28.
Cetin, I., Corbetta, C., Sereni, L. P., Marconi, A. M., Bozzetti, P., Pardi, G., & Battaglia, F. C. (1990). Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. American Journal of Obstetrics and Gynecology, 162(1), 253–261.
Dunn, W. B., Erban, A., Weber, R. J. M., Creek, D. J., Brown, M., Breitling, R., et al. (2012). Mass appeal: Metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics, 9(1), 44–66.
Efeyan, A., Zoncu, R., Chang, S., Gumper, I., Snitkin, H., Wolfson, R. L., et al. (2013). Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature, 493, 679–683.
Hawryluk, J., Grafka, A., Gęca, T., Dzida, G., & Łopucki, M. (2016). Study on free amino acid concentrations in umbilical cord blood plasma of newborns from normoglycaemic and gestational diabetes-complicated pregnancies. Journal of Pre-Clinical and Clinical Research, 10(1), 12–22.
Hu, R., Huang, D., Tong, J., Liao, Q., Hu, Z., & Ouyang, W. (2014). Aspartic acid in the hippocampus: a biomarker for postoperative cognitive dysfunction. Neural Regeneration Research, 9(2), 143–152.
Ivorra, C., García-Vicent, C., Chaves, F. J., Monleón, D., Morales, J. M., & Lurbe, E. (2012). Metabolomic profiling in blood from umbilical cords of low birth weight newborns. Journal of Translational Medicine, 10(1), 1–10.
Jao, J., & Abrams, E. J. (2014). Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. The Pediatric Infectious Disease Journal, 33(7), 734–740.
Kuma, A., Hatano, M., Matsui, M., Yamamoto, A., Nakaya, H., Yoshimori, T., et al. (2004). The role of autophagy during the early neonatal starvation period. Nature, 432, 1032–1036.
Levine, B. (2005). Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell, 120(2), 159–162.
Lum, J. J., Bauer, D. E., Kong, M., Harris, M. H., Li, C., Lindsten, T., & Thompson, C. B. (2005). Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell, 120(2), 237–248.
Marconi, A. M., Cetin, I., Davoli, E., Baggiani, A. M., Fanelli, R., Fennessey, P. V., et al. (1993). An evaluation of fetal glucogenesis in intrauterine growth-retarded pregnancies. Metabolism: Clinical and Experimental, 42(7), 860–864.
Mels, C., Jansen van Rensburg, P., van der Westhuizen, F. H., Pretorius, P. J., & Erasmus, E. (2011). Increased excretion of c4-carnitine species after a therapeutic acetylsalicylic acid dose: Evidence for an inhibitory effect on short-chain fatty acid metabolism. International Scholarly Research Network, 2011, 1–8.
Moutloatse, G. P., Bunders, M. J., van Reenen, M., Mason, S., Kuijpers, T. W., Engelke, U. F.H., & Reinecke, C. J. (2016). Metabolic risks at birth of neonates exposed in utero to HIV-antiretroviral therapy relative to unexposed neonates: An NMR metabolomics study of cord blood. Metabolomics, 12(175), 1–14.
Newell, M. L., & Bunders, M. J. (2013). Safety of antiretroviral drugs in pregnancy and breastfeeding for mother and child. Current Opinion in HIV and AIDS, 8(5), 504–510.
Philipps, A. F., Holzman, I. R., Teng, C., & Battaglia, F. C. (1978). Tissue concentrations of free amino acids in term human placentas. American Journal of Obstetrics and Gynecology, 131(8), 881–887.
Rashed, M. S., Ozand, P. T., Bucknall, M. P., & Little, D. (1995). Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatric Research, 38(3), 324–331.
Reinke, S. N., Walsh, B. H., Boylan, G. B., Sykes, B. D., Kenny, L. C., Murray, D. M., & Broadhurst, D. I. (2013). 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: Implications for hypoxic ischemic encephalopathy. Journal of Proteome Research, 12(9), 4230–4239.
Ritz-Timme, S., & Collins, M. J. (2002). Racemization of aspartic acid in human proteins. Ageing. Research Reviews, 1(1), 43–59.
Sanz-Cortés, M., Carbajo, R. J., Crispi, F., Figueras, F., Pineda-Lucena, A., & Gratacós, E. (2013). Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PloS One, 8(12), e80121.
Saugstad, O. D. (1975). Hypoxanthine as a measurement of hypoxia. Pediatric Research, 9(4), 158–161.
Shimizu, T., Watanabe, A., Ogawara, M., Mori, H., & Shirasawa, T. (2000). Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Archives of Biochemistry and Biophysics, 381(2), 225–234.
Soltesz, G., Harris, D., Mackenzie, I. Z., & Aynsley-Green, A. (1985). The metabolic and endocrine milieu of the human fetus and mother at 18–21 weeks of gestation. I. Plasma amino acid concentrations. Pediatric Research, 19(1), 91–93.
Tea, I., Le Gall, G., Küster, A., Guignard, N., Alexandre–Gouabau, M.-C., Darmaun, D., & Robins, R. J. (2012). 1 H-NMR-based metabolic profiling of maternal and umbilical cord blood indicates altered materno-foetal nutrient exchange in preterm infants. PLoS One, 7(1), 1–12.
Walsh, B. H., Broadhurst, D. I., Mandal, R., Wishart, D. S., Boylan, G. B., Kenny, L. C., & Murray, D. M. (2012). The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS One, 7(12), e50520.
Zoncu, R., Efeyan, A., & Sabatini, D. M. (2011). mTOR: From growth signal integration to cancer, diabetes and ageing. Nature Reviews of Molecular and Cell Biology, 12(1), 21–35.
Acknowledgements
We thank Dr. K. Boer, Prof. T. W. Kuijpers, Dr. M. Godfried, Dr. J. Nellen, Dr. E. van Leeuwen and Dr. H. J. Scherpbier for caring for the pregnant women and their infants and collection of cord blood samples. This work was supported by the Netherlands Organisation for Scientific Research through an Agiko stipendium (92003417) and the Dutch AIDS Foundation (2006015). Further support for this research was provided by the Technological Innovation Agency (TIA) of the Department of Science and Technology of South Africa. We also acknowledge the National Research Foundation (NRF) of South Africa for awarding GM a postgraduate busary. The views and findings of this investigation are those of the authors and do not reflect or represent any policies of the funders.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Neonates: Ethical approval for the study and for the use of the samples from the two neonatal groups was obtained from the Ethics Committee of the Academic Medical Centre at the University of Amsterdam. Infants: Ethical approval for the samples from the infant group was obtained from the Ethics Committees of North-West University (Reference No. 02M02), including informed consent from the parents for serum analyses and eventual publication of results from anonymous cases in accordance with the ethical requirements of the diagnostic laboratory (Potchefstroom Laboratory for Inborn Errors of Metabolism).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moutloatse, G.P., Schoeman, J.C., Lindeque, Z. et al. Metabolic risks of neonates at birth following in utero exposure to HIV-ART: the amino acid profile of cord blood. Metabolomics 13, 89 (2017). https://doi.org/10.1007/s11306-017-1222-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1222-y