Abstract
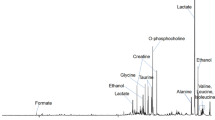
In order to elucidate the metabolite changes associated with hepatocellular carcinoma (HCC) oncogenesis and progression, we compared the profiles obtained by 1D proton HRMAS NMR spectroscopy of 51 needle biopsies (14 primary nodules, 14 recurrent, and 23 paired cirrhotic specimens). The diagnosis of HCC was based on 2 concordant imaging techniques and was confirmed by histology in 20 cases. Spectroscopy was performed using a Bruker AVANCE II 600 spectrometer. One-dimensional proton spectra were acquired using water-suppressed (noesygppr) pulse and spin-echo CPMG sequences. Signals were assigned by BBIOREFCODE and were confirmed by HSQC. Statistics was based on the SIMCA P package. Orthogonal projection to latent structure (OPLS-DA) showed a clear separation between tumor and cirrhosis. This difference was maintained when the analysis of paired samples from primary to recurrent nodules was split. OPLS-DA of primary and recurrent nodules also showed a significant difference. The relationship between metabolite profile and HCC volume was evaluated comparing the spectra obtained in tumors ≤2 cm (n = 15) and in those larger than 2 cm (n = 11). Univariate comparison of the most relevant metabolites showed that: (1) increased choline, TMAO, and decreased saturated fatty acids differentiate HCC from the surrounding tissue; (2) increased lactate and myo-inositol differentiate recurrent from primary HCC; (3) decreased saturated fatty acids characterize large HCC nodules.




Similar content being viewed by others
References
Altekruse, S. F., McGlynn, K. A., Dickie, L. A., & Kleiner, D. E. (2012). Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology, 55(2), 476–482.
Beyoğlu, D., Imbeaud, S., Maurhofer, O., et al. (2013). Tissue metabolomics of hepatocellular carcinoma: Tumor energy metabolism and the role of transcriptomic classification. Hepatology,. doi:10.1002/hep.26350.
Boyault, S., Rickman, D. S., de Reyniès, A., et al. (2007). Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology, 45(1), 42–52.
Budhu, A., Roessler, S., Zhao, X., et al. (2013). Integrated metabolite and gene expression profiles identify lipid biomarkers associated with progression of hepatocellular carcinoma and patient outcomes. Gastroenterology, 144(5), 1066–1075.
Bylesjö, M., Rantalainen, M., Cloarec, O., Nicholson, J. K., Holmes, E., & Trygg, J. (2006). OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. Journal of Chemometrics, 20, 341–351.
Calvisi, D. F., Factor, V. M., & Ladu, S. (2004). Disruption of beta-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology, 126, 1374–1386.
Calvisi, D. F., Wang, C., Ho, C., et al. (2011). Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology, 140(3), 1071–1083.
Chun, J. M., Kwon, H. J., Sohn, J., et al. (2011). Prognostic factors after early recurrence in patients who underwent curative resection for hepatocellular carcinoma. Journal of Surgical Oncology, 103(2), 148–151.
Cobbold, J. F. L., & Patel, J. H. (2010). Hepatic lipid profling in chronic hepatitis C: an in vitro and in vivo proton magnetic resonance spectroscopy study. Journal of Hepatology, 52, 16–24.
Forner, A., Llovet, J. M., & Bruix, J. (2012). Hepatocellular carcinoma. Lancet, 379, 1245–1255.
Galons, J. B., Job, C., & Gilles, R. (1995). Increase of GPC levels in cultured mammalian cells during acidosis. Magnetic Resonance in Medicine, 33, 422–426.
Glund, K., Bhujiwalla, Z. M., & Rohen, S. M. (2011). Choline metabolism in malignant transformation. Nature Reviews Cancer, 11, 835–848.
Gogvadze, V., Zhivotovski, B., & Orrenius, S. (2009). The Warburg effect and mitochondrial stability in cancer cell. Molecular Aspects of Medicine, 31, 60–74.
Griffin, J. L., & Shockcor, J. P. (2004). Metabolic profiles of cancer cells. Nature Reviews Cancer, 4, 551–561.
Iavarone, F., Sangiovanni, A., & Forzenigo, L. V. (2010). Diagnosis of hepatocellular carcinoma in cirrhosis by dynamic contrast imaging: the importance of tumor cell differentiation. Hepatology, 52(5), 1723–1730.
Livraghi, T., Meloni, F., Di Stasi, M., et al. (2008). Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology, 47(1), 82–89.
López-Lázaro, M. (2008). The Warburg effect: Why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents in Medicinal Chemistry, 8, 305–312.
Martinez-Granados, B., Monleon, D., Martinez-Bisbal, M. C., et al. (2006). Metabolite identification in human liver needle biopsies by high-resolution magic angle spinning 1H NMR spectroscopy. NMR in Biomedicine, 19, 90–100.
Minquez, B., Hoshida, Y., & Villanueva, A. (2011). Gene-expressione signature of vascular invasion in hepatocellular carcinoma. Journal of Hepatology, 55(6), 1325–1331.
Negrini, S., Gorgoulis, V. G., & Halozenetis, T. D. (2010). Genomic instability- an evolving hallmark of cancer. Nature Reviews Molecular Cell Biology, 11, 220–228.
Ng, D. J. Y., Pasikanti, K. K., & Chan, E. C. Y. (2011). Trend analysis of Metabolomics and systematic review of metabolomics-derived cancer marker metabolites. Metabolomics, 7, 155–178.
Paris, D., Melck, D., Stocchero, M., D’Apolito, O., Calemma, R., Castello, G., et al. (2010). Monitoring liver alterations during hepatic tumorigenesis by NMR profiling and pattern recognition. Metabolomics, 6, 405–416.
Park, Y., Choi, D., Lim, H. K., et al. (2008). Growth rate of new hepatocellular carcinoma after percutaneous radiofrequency ablation: evaluation with multiphase CT. American Journal of Roentgenology, 191(1), 215–220.
Rimola, J., Forner, A., Tremosini, S., et al. (2012). Non invasive diagnosis of hepatocellular carcinoma ≤2 cm in cirrhosis. Diagnostic accuracy. Journal of Hepatology, 56, 1317–1323.
Roayaie, S., Obeidat, K., Sposito, C., et al. (2013). Resection of hepatocellular cancer ≤2 cm: Results from two western centers. Hepatology,. doi:10.1002/hep.25832.
Sangiovanni, A., Del Ninno, E., Fasani, P., et al. (2004). Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology, 126, 1005–1014.
Trygg, J., & Wold, S. (2002). Orthogonal projections to latent structures, O-PLS. Journal of Chemometrics, 16, 119–128.
Wiklund, S., Johansson, E., Sjöström, L., et al. (2008). Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Analytical Chemistry, 80(1), 115–122.
Wishart, D. S., Jewison, T., Guo, A. C., et al. (2013). HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Research, 41(Database issue), D801–D807.
Yang, Y., Li, C., Nie, X., & Feng, X. (2007). Metabonomic studies of human hepatocellular carcinoma using high resolution magic angle spinning. Journal of Proteome Research, 6, 2605–2614.
Zhang, B., Yang, B. H., & Tang, Z. Y. (2004). Randomized controlled trial of screening for hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology, 130(7), 417–422.
Acknowledgments
The authors wish to thank Dr. F. Benevelli, Dr. A. Minoja and Dr. C. Napoli (Bruker Italy) for suggestions and discussions and Dr. L. Barberini (University of Cagliari) for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solinas, A., Chessa, M., Culeddu, N. et al. High resolution-magic angle spinning (HR-MAS) NMR-based metabolomic fingerprinting of early and recurrent hepatocellular carcinoma. Metabolomics 10, 616–626 (2014). https://doi.org/10.1007/s11306-013-0601-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-013-0601-2