Abstract
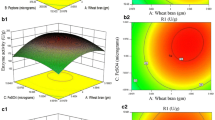
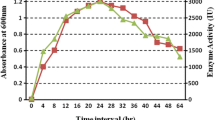
α-Galactosidase is an important exoglycosidase belonging to the hydrolase class of enzymes, which has therapeutic and industrial potential. It plays a crucial role in hydrolyzing α-1,6 linked terminal galacto-oligosaccharide residues such as melibiose, raffinose, and branched polysaccharides such as galacto-glucomannans and galactomannans. In this study, Actinoplanes utahensis B1 was explored for α-galactosidase production, yield improvement, and activity enhancement by purification. Initially, nine media components were screened using the Plackett–Burman design (PBD). Among these components, sucrose, soya bean flour, and sodium glutamate were identified as the best-supporting nutrients for the highest enzyme secretion by A. Utahensis B1. Later, the Central Composite Design (CCD) was implemented to fine-tune the optimization of these components. Based on sequential statistical optimization methodologies, a significant, 3.64-fold increase in α-galactosidase production, from 16 to 58.37 U/mL was achieved. The enzyme was purified by ultrafiltration-I followed by multimode chromatography and ultrafiltration-II. The purity of the enzyme was confirmed by Sodium Dodecyl Sulphate–Polyacrylamide Agarose Gel Electrophoresis (SDS-PAGE) which revealed a single distinctive band with a molecular weight of approximately 72 kDa. Additionally, it was determined that this process resulted in a 2.03-fold increase in purity. The purified α-galactosidase showed an activity of 2304 U/mL with a specific activity of 288 U/mg. This study demonstrates the isolation of Actinoplanes utahensis B1 and optimization of the process for the α-galactosidase production as well as single-step purification.
Graphical abstract





Similar content being viewed by others
Data availability
All data analysed in this study is included in this article.
References
Al-Dhabaan FA (2019) Morphological, biochemical and molecular identification of petroleum hydrocarbons biodegradation bacteria isolated from oil polluted soil in Dhahran. Saud Arabia Saudi J Biological Sciences 26:1247–1252
Aleksieva P, Tchorbanov B, Nacheva L (2010) High-yield production of α-galactosidase excreted from Penicillium chrysogenum and Aspergillus niger. Biotechnol Biotechnol Eq 24:1620–1623
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Alvarez-Cao ME, Cerdan ME, Gonzalez-Siso MI, Becerra M (2019) Optimization of Saccharomyces cerevisiae α-galactosidase production and application in the degradation of raffinose family oligosaccharides. Microb Cell Fact 18:172
Anisha GS, Sukumaran RK, Prema P et al (2008) Statistical Optimization of α-Galactosidase Production in Submerged Fermentation by Streptomyces griseoloalbus Using Response Surface Methodology. Food Technol Biotechnol 46(2):171–177
Balabanova LA, Bakunina IY, Nedashkovskaya OI et al (2010) Molecular characterization and therapeutic potential of a marine bacterium Pseudoalteromonas sp. KMM 701 α-galactosidase. Mar Biotechnol (NY) 12:111–120
Bhatia S, Singh A, Batra N, Singh J (2020) Microbial production and biotechnological applications of α-galactosidase. Int J Biol Macromol 150:1294–1313
Boje BG, Narasu ML, Chakravarthy BK, Savala NK (2011) Optimization of production conditions for intracellular α-galactosidase from Enterobacter dissolvens. Int J Biol Sci II:155–161
Brouns SJ, Smits N, Wu H, Snijders APL, Wright PC, de Vos WM (2006) Identification of a novel α-galactosidase from the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol 88:2392–2399
Buchanan RE, Gibbons NE (1974) Bergey’s manual of determinative bacteriology, 8th edn. Williams & Wilkins Co., Baltimore
Cazetta ML, Celligoi MAPC, Buzato JB, Scarmino IS (2007) Fermentation of molasses by Zymomonas mobilis: Effects of temperature and sugar concentration on ethanol production. Biores Technol 98:2824–2828
Chauhan AS, Kumar A, Siddiqi NJ, Sharma B (2015) Extracellular α-galactosidase from Trichoderma sp.(WF-3): optimization of enzyme production and biochemical characterization. Biotechnol Res Int 2015:860343
Chen M, Mustapha A (2012) Survival of freeze-dried microcapsules of α-galactosidase producing probiotics in a soybar matrix. Food Microbiol 30:68–73
Chiranjeevi PV, Pandian MR, Sathish T et al (2014) Enhancement of laccase production from Plerotus ostreatus PVcRSP-7 by altering the nutritional conditions using response surface methodology. Bioresources 9(3):4212–4225
Clarke JH, Davidson K, Rixon JE, Halstead JR, Fransen MP, Gilbert HJ, Hazlewood GP (2000) A comparison of enzyme-aided bleaching of softwood paper pulp using combinations of xylanase, mannanase and a-galactosidase. Appl Microbiol Biotechnol 53:661–667
Clarridge JE (2004) Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev 4:840–862
Darby AC, Chandler SM, Welburn SC, Douglas AE (2005) Aphid-symbiotic bacteria cultured in insect cell lines. Appl Environ Microbiol 71(8):4833–4839
Dey PM, Pridham JB (1969) Purification and properties of a-galactosidases from Vicia faba seeds. Biochem J 113:49–55
Dey PM, Pridham JB (1972) Biochemistry of a-galactosidase. Adv Enzymol 36:911–930
De Rezende ST, Guimaraes VM, Rodrigues MC, Felix CR (2005) Purification and Characterization of an α-Galactosidase from Aspergillus fumigate. Braz Arch Biol Technol 48:195–202
Francis F, Sabu A, Nampoothiri KM, Ramachandran S, Ghosh S, Szakacs G, Pandey A (2003) Use of response surface methodology for optimizing process parameters for the production of a-amylase by Aspergillus oryzae. Biochem Eng J 15:107–115
Gajdhane SB, Bhagwat PK, Dandge PB (2016) Response surface methodology- based optimization of production media and purification of α-galactosidase in solid-state fermentation by Fusarium moniliforme NCIM 1099. 3 Biotech 6:1–14
Garro MS, de Valdez GF, Oliver G, de Gori GS (1996) Purification of α-galactosidase from Lactobacillus fermentum. J Biotechnol 45:103–109
Gote MM, Khan MI, Gokhale DV, Bastawde KB, Khire JM (2006) Purification, characterization and substrate specificity of thermostable α-galactosidase from Bacillus stearothermophilus (NCIM-5146). Process Biochem 41:1311–1317
Holt JG (1994) Bergey’s manual of determinative bacteriology, 9th edn. Lippincott Williams and Wilkins, Baltimore
Kang JJ, Desch KC, Kelly RJ, Shu L, Bodary PF, Shayman JA (2019) α-Galactosidase-A deficiency promotes von Willebrand factor secretion in models of Fabry disease. Kidney Int 95:149–159
Kennedy M, Krouse D (1999) Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biotechnol 23:456–475
Kuster E, Williams ST (1964) Selection of media for isolation of streptomycetes. Nature 202:928–929
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Liu QP, Sulzenbacher G, Yuan H et al (2007) Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol 25:454–464
Mohan SK, Viruthagiri T, Arunkumar C (2014) Statistical optimization of process parameters for the production of tannase by aspergillus flavus under submerged fermentation. 3 Biotech 4:159–166
Naganagouda K, Mulimani VH (2006) Gelatin blends with alginate: gel fibers for α-galactosidase immobilization and its application in reduction of non-digestible oligosaccharides in soymilk. Process Biochem 41:1903–1907
Panda BP, Ali M, Javed S (2007) Fermentation process optimization. Res J Microbiol 2:201–208
Patil AG, Kote NV, Manjula AC, Vishwanatha T (2021) Purification, characterization of α-galactosidase from a novel Bacillus megaterium VHM1, and its applications in the food industry. J Appl Biol Biotechnol 9:13–19
Saishin N, Ueta M, Wada A, Yamamoto I (2010) Purification and characterization of α-galactosidase I from Bifidobacterium longum subsp. longum JCM 7052. J Biol Micromol 10:13–22
Sathish T, Kezia D, Bramhachari PV, Prakasham RS (2018) Multi-objective based superimposed optimization method for enhancement of l-glutaminase production by Bacillus subtilis RSP-GLU. Karbala Int J of Modern Sci 4(1):50–60
Schroder C, Janzer VA, Schirrmacher G, Claren J, Antranikian G (2017) Characterization of two novel heat-active α-galactosidases from thermophilic bacteria. Extremophiles 21:85–94
Shirling G (1966) Methods of characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Shibuya H, Kobayashi H, Sato T et al (1997) Purification, characterization and cDNA cloning of a novel α-galactosidase from Mortierella vinacea. Biosci Biotechnol Biochem 61:592–598
Sirisha E, Potumarthi R, Naveen A, Mangamoori LN (2015) Purification and characterisation of intracellular alpha-galactosidases from Acinetobacter sp. 3 Biotech 5:925–932
Stratilova B, Klaudiny J, Rehulka P et al (2018) Characterization of a long-chain α-galactosidase from Papiliotrema flavescens. World J Microbiol Biotechnol 34:19
Thippeswamy S, Mulimani VH (2002) Enzymatic degradation of raffinose family oligosaccharides in soymilk by immobilized α-galactosidase from Gibberella fujikuroi. Process Biochem 38:635–640
Usman AI, Aziz AA, Sodipo BK (2019) Application of central composite design for optimization of biosynthesized gold nanoparticles via sonochemical method. SN Applied Sciences 1:403
Waterborg JH, Walker, (2009) The Lowry method for protein quantitation. In: Walker JM (ed) The Protein Protocols Handbook. Humana Press, Totowa, NJ, pp 7–10
Weignerova L, Simerska P, Kren V (2009) α-Galactosidases and their applications in biotransformations. Biocatal Biotransform 27:79–89
Zeyland J, Wozniak A, Gawronska B et al (2014) Double Transgenic pigs with Combined expression of human α1,2-Fucosyltransferase and α-galactosidase to avoid hyperacute xenograft rejection. Arch Immunol Ther Exp 62:411–422
Acknowledgements
The authors express their gratitude to Dr. Harish Kumar Reddy, Manufacturing Science Department at Biological E Ltd for generously providing the laboratory to conduct the study. The authors would like to thank Dr. Sathish Thadikamala, Aurovaccines, Hyderabad, TS, India for his support in conducting statistical studiesas well as support during the drafting of this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
BK and JBD: conceptualized, designed and wrote the manuscript. VTC: revised the manuscript and also major contributor in writing the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Balumahendra, K., Venkateswarulu, T.C. & Babu, D.J. Enhancement of α-galactosidase production using novel Actinoplanes utahensis B1 strain: sequential optimization and purification of enzyme. World J Microbiol Biotechnol 40, 91 (2024). https://doi.org/10.1007/s11274-023-03880-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03880-1