Abstract
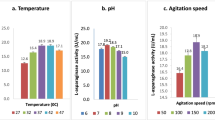
L-asparaginase is used as one of the prime chemotherapeutic agents to treat acute lymphoblastic leukemia. L-asparaginase obtained from bacteria exhibits hypersensitive reactions including various side effects. The present work aimed to optimize growth parameters for maximum production of L-asparaginase by Fusarium foetens through response surface methodology, its purification, and characterization. The optimization of L-asparaginase production by Fusarium foetens was initially done through a one-factor-at-a-time method. L-asparaginase production was further optimized using a central composite design based response surface methodology. The maximum L-asparaginase activity of 12.83 IU/ml was obtained under the following growth conditions; temperature-27.5 °C, pH-8, inoculum concentration-1.5 × 106 spores/ml, and incubation period-7 days. In comparison with the unoptimized growth conditions (4.58 IU/ml), the optimization led to a 2.65-fold increase in the L-asparaginase activity. The L-asparaginase from Fusarium foetens was purified 15.60-fold, with a yield of 39.89% using DEAE-cellulose column chromatography. After purification, the L-asparaginase activity was determined to be 127.26 IU/ml and the specific activity was found to be 231.38 IU/mg. The molecular mass was estimated to be approximately 37 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The purified enzyme showed optimum activity at pH 5, and a temperature of 40 °C. The enzyme showed 100% specificity towards L-asparagine and no activity towards L-glutamine. Its activity was enhanced by Mn2+, Fe2+, and Mg2, while it was inhibited by β-mercaptoethanol and EDTA. The Km and Vmax of the purified L-asparaginase were found to be 23.82 mM and 210.3 IU/ml respectively. The results suggest that Fusarium foetens could be a potent candidate for the bioprocessing of L-asparaginase at a large scale.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Abbas Ahmed MM, Nageh Abo Dahab F, Taher Taha M, Fareed Hassan SM (2015) Production, purification and characterization of L-asparaginase from marine endophytic aspergillus sp. ALAA-2000 under submerged and solid state fermentation. J Microb Biochem Technol 7:165–172
Abdel-Rahman MA, Hassan SED, El-Din MN et al (2020) One-factor-at-a-time and response surface statistical designs for improved lactic acid production from beet molasses by Enterococcus hirae ds10. SN Appl Sci 2:1–14
Abhini KN, Rajan AB, Fathimathu Zuhara K, Sebastian D (2022) Response surface methodological optimization of l-asparaginase production from the medicinal plant endophyte Acinetobacter baumannii ZAS1. J Genet Eng Biotechnol 20:1–13
Al Yousef SA (2022) Fusarium sp. L-asparaginases: purification, characterization, and potential assessment as an antileukemic chemotherapeutic agent. Environ Sci Pollut Res 29:11243–11254
Alam S, Pranaw K, Tiwari R, Khare SK (2019) Recent development in the uses of asparaginase as food enzyme. Green bio-processes. Springer, pp 55–81
Alrumman SA, Mostafa YS, Al-Izran KA et al (2019) Production and anticancer activity of an L-asparaginase from Bacillus licheniformis isolated from the Red Sea, Saudi Arabia. Sci Rep 9:1–14
Araújo-Magalhães GR, Maciel MHC, da Silva LF et al (2021) Fungal endophytes from leaves of Mandevilla catimbauensis (Apocynaceae): diversity and potential for L-asparaginase production. Braz J Microbiol 52:1431–1441
Asgher M, Arshad S, Qamar SA, Khalid N (2020) Improved biosurfactant production from Aspergillus niger through chemical mutagenesis: characterization and RSM optimization. SN Appl Sci 2:1–11
Ashok A, Devarai SK (2019) l-Asparaginase production in rotating bed reactor from Rhizopus microsporus IBBL-2 using immobilized Ca-alginate beads. 3 Biotech 9:1–10
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breig SJM, Luti KJK (2021) Response surface methodology: A review on its applications and challenges in microbial cultures. In: Materials Today: Proceedings
Chand S, Mahajan RV, Prasad JP et al (2020) A comprehensive review on microbial l-asparaginase: Bioprocessing, characterization, and industrial applications. Biotechnol Appl Biochem 67:619–647
Chang S-W, Shieh C-J, Lee G-C et al (2006) Optimized growth kinetics of Pichia pastoris and recombinant Candida rugosa LIP1 production by RSM. Microb Physiol 11:28–40
Chi H, Chen M, Jiao L et al (2021) Characterization of a novel L-Asparaginase from Mycobacterium gordonae with acrylamide mitigation potential. Foods 10:2819
Costa-Silva TA, Camacho-Córdova DI, Agamez-Montalvo GS et al (2019) Optimization of culture conditions and bench-scale production of anticancer enzyme L-asparaginase by submerged fermentation from aspergillus terreus CCT 7693. Prep Biochem Biotechnol 49:95–104
da Cunha MC, Silva LC, Sato HH, de Castro RJS (2018) Using response surface methodology to improve the L-asparaginase production by Aspergillus niger under solid-state fermentation. Biocatal Agric Biotechnol 16:31–36
da Cunha MC, Aguilar JG dos, Orrillo Lindo S et al (2022) SMDR, L-asparaginase from Aspergillus oryzae spp.: effects of production process and biochemical parameters. Prep Biochem Biotechnol 52:253–263
Da Rocha WRV, Costa-Silva TA, Agamez‐Montalvo GS et al (2019) Screening and optimizing fermentation production of l‐asparaginase by aspergillus terreus strain S‐18 isolated from the brazilian Caatinga Biome. J Appl Microbiol 126:1426–1437
Dange V, Peshwe S (2015) Purification and biochemical characterization of L-asparaginase from Aspergillus niger and evaluation of its antineoplastic activity. Int J Sci Res 4:564–569
Dias FFG, Sato HH (2016) Sequential optimization strategy for maximum l-asparaginase production from Aspergillus oryzae CCT 3940. Biocatal Agric Biotechnol 6:33–39
Dias FFG, Ruiz ALTG, Della Torre A, Sato HH (2016) Purification, characterization and antiproliferative activity of L-asparaginase from Aspergillus oryzae CCT 3940 with no glutaminase activity. Asian Pac J Trop Biomed 6:785–794
Diwaniyan S, Sharma KK, Kuhad RC (2012) Laccase from an alkalitolerant basidiomycetes Crinipellis sp. RCK-1: production optimization by response surface methodology. J Basic Microbiol 52:397–407
Doriya K, Kumar DS (2018) Optimization of solid substrate mixture and process parameters for the production of L-asparaginase and scale-up using tray bioreactor. Biocatal Agric Biotechnol 13:244–250
Dutta S, Ghosh S, Pramanik S (2015) L-asparaginase and L-glutaminase from aspergillus fumigatus WL002: production and some physicochemical properties. Appl Biochem Microbiol 51:425–431
Ekpenyong M, Asitok A, Antigha R et al (2021) Bioprocess optimization of nutritional parameters for enhanced anti-leukemic L-asparaginase production by Aspergillus candidus UCCM 00117: a sequential statistical approach. Int J Pept Res Ther 27:1501–1527
El-Gendy MMAA, Awad MF, El-Shenawy FS, El-Bondkly AMA (2021) Production, purification, characterization, antioxidant and antiproliferative activities of extracellular L-asparaginase produced by Fusarium equiseti AHMF4. Saudi J Biol Sci 28:2540–2548
El-Naggar NE, El-Ewasy SM, El-Shweihy NM (2014) Microbial L-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: the pros and cons. Int J Pharmacol 10:182–199
El-Refai HA, Shafei MS, Mostafa H et al (2016) Comparison of free and immobilized L-asparaginase synthesized by gamma-irradiated Penicillium cyclopium. Pol J Microbiol 65:3
Elshafei AM, Hassan MM, Abouzeid MA-E et al (2012) Purification, characterization and antitumor activity of L-asparaginase from Penicillium brevicompactum NRC 829. Br Microbiol Res J 2:158
Erva RR, Venkateswarulu TC, Pagala B (2018) Multi level statistical optimization of l-asparaginase from Bacillus subtilis VUVD001. Biotech 8(3):24
Fazeli N, Alimadadi N, Nasr S (2021) Screening and optimization of process parameters for the production of l-asparaginase by indigenous fungal-type strains. Iran J Sci Technol Trans A 45:409–416
Fonseca MHG, da Silva Fiúza T, de Morais SB, Trevizani R (2021) Circumventing the side effects of L-asparaginase. Biomed Pharmacother 139:111616
Ghosh S, Murthy S, Govindasamy S, Chandrasekaran M (2013) Optimization of L-asparaginase production by Serratia marcescens (NCIM 2919) under solid state fermentation using coconut oil cake. Sustain Chem Process 1:1–8
Golbabaie A, Nouri H, Moghimi H, Khaleghian A (2020) l-asparaginase production and enhancement by Sarocladium strictum: in vitro evaluation of anti‐cancerous properties. J Appl Microbiol 129:356–366
Hassan SWM, Farag AM, Beltagy EA (2018) Purification, characterization and anticancer activity of L-asparaginase produced by marine aspergillus terreus. J Pure Appl Microbiol 12:1845–1854
Imada A, Igarasi S, Nakahama K, Isono M (1973) Asparaginase and glutaminase activities of micro-organisms. Microbiol (N Y) 76:85–99
Irfan M, Nadeem M, Syed Q (2014) One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride-IR05 in solid-state fermentation. J Radiat Res Appl Sci 7:317–326
Khalil NM, Rodríguez-Couto S, El-Ghany MNA (2021) Characterization of Penicillium crustosum L-asparaginase and its acrylamide alleviation efficiency in roasted coffee beans at non-cytotoxic levels. Arch Microbiol 203:2625–2637
Kheiralla ZH, El-Gendy NS, Ahmed HA et al (2018) One-factor-at-a-time (OFAT) optimization of hemicellulases production from Fusarium moniliforme in submerged fermentation. Energy Sources A: Recovery Util Environ Eff 40:1877–1885
Killander D, Dohlwitz A, Engstedt L et al (1976) Hypersensitive reactions and antibody formation during L-asparaginase treatment of children and adults with acute leukemia. Cancer 37:220–228
Kumar S, Dasu VV, Pakshirajan K (2011) Purification and characterization of glutaminase-free L-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour Technol 102:2077–2082
Kumar NSM, Ramasamy R, Manonmani HK (2013) Production and optimization of L-asparaginase from Cladosporium sp. using agricultural residues in solid state fermentation. Ind Crops Prod 43:150–158
Kumar V, Ahluwalia V, Saran S et al (2021) Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour Technol 323:124566
Kwon YM, Chung HS, Moon C et al (2009) L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL). J Control Release 139:182–189
Laemmli UK (1970) SDS-page Laemmli method. Nature 227:680–685
Loureiro CB, Borges KS, Andrade AF et al (2012) Purification and biochemical characterization of native and pegylated form of L-asparaginase from Aspergillus terreus and evaluation of its antiproliferative activity
Meghavarnam AK, Janakiraman S (2015) Purification and characterization of therapeutic enzyme L-asparaginase from a tropical soil fungal isolate Fusarium culmorum ASP-87. J Anesth Crit Care Open Access 2:64
Meghavarnam AK, Janakiraman S (2017) Solid state fermentation: an effective fermentation strategy for the production of L-asparaginase by Fusarium culmorum (ASP-87). Biocatal Agric Biotechnol 11:124–130
Mesas JM, Gil JA, Martn JF (1990) Characterization and partial purification of L-asparaginase from Corynebacterium glutamicum. Microbiol (N Y) 136:515–519
Mohan Kumar NS, Manonmani HK (2013) Purification, characterization and kinetic properties of extracellular L-asparaginase produced by Cladosporium sp. World J Microbiol Biotechnol 29:577–587
Monica T, Lincoln L, Niyonzima FN, Sunil SM (2013) Isolation, purification and characterization of fungal extracellular L-Asparaginase from Mucor Hiemalis. J Biocatal Biotransformation 2: 2 of 9:12–14
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419:448–449
Nunes JCF, Cristóvão RO, Freire MG et al (2020) Recent strategies and applications for l-Asparaginase confinement. Molecules 25:5827
Nwabueze TU (2010) Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. Int J Food Sci Technol 45:1768–1776
Rispoli FJ, Shah V (2007) Mixture design as a first step for optimization of fermentation medium for cutinase production from Colletotrichum lindemuthianum. J Ind Microbiol Biotechnol 34:349–355
Saeed H, Ali H, Soudan H et al (2018) Molecular cloning, structural modeling and production of recombinant aspergillus terreus L. asparaginase in Escherichia coli. Int J Biol Macromol 106:1041–1051
Saha SP, Mazumdar D (2019) Optimization of process parameter for alpha-amylase produced by Bacillus cereus amy3 using one factor at a time (OFAT) and central composite rotatable (CCRD) design based response surface methodology (RSM). Biocatal Agric Biotechnol 19:101168
Saleena SK, Johnson JI, Joseph JK et al (2022) Production and optimization of l-asparaginase by Streptomyces koyangensis SK4 isolated from Arctic sediment. J Basic Microbiol
Shafei MS, El-Refai HA, Mostafa H et al (2015) Purification, characterization and kinetic properties of Penicillium cyclopium L-asparaginase: impact of L-asparaginase on acrylamide content in potato products and its cytotoxic activity. Curr Trends Biotechnol Pharm 9:132–140
Singh Y, Gundampati RK, Jagannadham MV, Srivastava SK (2013) Extracellular L-asparaginase from a protease-deficient Bacillus aryabhattai ITBHU02: purification, biochemical characterization, and evaluation of antineoplastic activity in vitro. Appl Biochem Biotechnol 171:1759–1774
Singh V, Haque S, Niwas R et al (2017) Strategies for fermentation medium optimization: an in-depth review. Front Microbiol 7:2087
Souza PM, de Freitas MM, Cardoso SL et al (2017) Optimization and purification of L-asparaginase from fungi: a systematic review. Crit Rev Oncol Hematol 120:194–202
Thakur M, Lincoln L, Niyonzima FN, Sunil SM (2014) Biotransformation isolation, purification and characterization of fungal extracellular L-Asparaginase from Mucor hiemalis. J Biocatal Biotransformation 2:1–9
Vala AK, Sachaniya B, Dudhagara D et al (2018) Characterization of L-asparaginase from marine-derived aspergillus niger AKV-MKBU, its antiproliferative activity and bench scale production using industrial waste. Int J Biol Macromol 108:41–46
Yadav N, Sarkar S (2014) Production of L-asparaginase by Fusarium oxysporum using submerged fermentation. Int J Pharm Sci Invent 3:32–40
Yap LS, Lee WL, Ting ASY (2021) Optimization of L-asparaginase production from endophytic Fusarium proliferatum using OFAT and RSM and its cytotoxic evaluation. J Microbiol Methods 191:106358
Zhao X, Kong X, Hua Y et al (2008) Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. ur J Lipid Sci Technol 110:405–412
Zuo S, Xue D, Zhang T et al (2014) Biochemical characterization of an extremely thermostable l-asparaginase from Thermococcus gammatolerans EJ3. J Mol Catal B Enzym 109:122–129
Zuo S, Zhang T, Jiang B, Mu W (2015) Reduction of acrylamide level through blanching with treatment by an extremely thermostable L-asparaginase during french fries processing. Extremophiles 19:841–851
Acknowledgements
The authors are thankful to the Council of Scientific and Industrial Research (CSIR) for providing a Junior Research Fellowship (JRF) and DOS in Microbiology, University of Mysore for providing facilities.
Funding
The study was funded by CSIR (Council of Scientific and Industrial Research) under Junior Research Fellowship (File No:09/119(0218)/2019-EMR-I).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Javaraiah Parashiva. The first draft of the manuscript was written by Javaraiah Parashiva and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parashiva, J., Nuthan, B.R., Bharatha, M. et al. Response surface methodology based optimized production, purification, and characterization of L-asparaginase from Fusarium foetens. World J Microbiol Biotechnol 39, 252 (2023). https://doi.org/10.1007/s11274-023-03684-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03684-3