Abstract
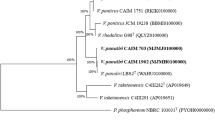
Molecular polymorphisms in a selected set of Spirulina and related genera using random primers based on repetitive sequences along with biochemical parameters, led to the unambiguous differentiation of the strains and understanding of their phylogenetic relationships. A combination of 10 sets of dual primers generated 100% distinct polymorphic bands ranging from 150 to 5,000 bp. Total number of fragments ranged from 68 to 159 whereas polymorphic bands ranged from 13 to 32 for different Random Amplified Polymorphic DNA (RAPD) reactions. Spirulina platensis strains, Sp-2 and Sp-3, possessed quite comparable chlorophyll and protein content besides having maximum similarity coefficient (0.88) between them on the basis of RAPD reactions, thus proved to be closely related. Sp-8 (Spirulina from Loktak Lake) having the highest protein content and protein: chlorophyll ratio, showed close similarity with the mutant of Spirulina platensis (Sp-7) on the basis of RAPD analysis. Duncan’s Multiple Range Test (DMRT) ranking for the biochemical parameters were quite closer for the strains of Spirulina and Arthrospira. This is also supported by the cluster analysis based on RAPD data, as the strains of Spirulina and Arthrospira are placed together in the same subcluster in the dendrogram. The comparative closeness among the strains of Lyngbya, Oscillatoria and Phormidium is reflected by the low content of protein and protein: chlorophyll ratio, which is also supported by the dendrogram based upon RAPD; thus, exhibiting the usefulness of multiplex RAPD along with biochemical parameters for the phylogenetic analysis of Spirulina and related genera.


Similar content being viewed by others
References
Anagnostidis K, Komarek J (1985) Modern approach to the classification system of cyanophytes 1—introduction. Arch Hydrobiol Suppl 71:291–302
Apte SK, Bhagwat AA (1989) Salinity stress induced proteins in two nitrogen-fixing Anabaena strains differently tolerant to salt. J Bacteriol 171:909–915
Bartley GE, Scolnick PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction and human health. Plant Cell 7:1027–1038
Boone DR, Castenholz RW (2001) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, p 721
Borowitzka MA (1988) Vitamins and fine chemicals from microalgae. In: Borowitzka MA, Borowitzka LJ (eds) Microalgal biotechnology. Cambridge University Press, Cambridge, pp 153–196
Burja AM, Banaigs B, Abou-Mansour E, Burgess JG, Wright PC (2001) Marine cyanobacteria—a prolific source of natural products. Tetrahedron 57:9347–9377
Castenholz RW (1989) Subsection IV. Order Nostocales. In: Staley JT, Bryant MP, Pfenning N, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 3. Williams and Wilkins, Baltimore, pp 1780–1793
Castenholz RW (1992) Species usage, concept, and evolution in the cyanobacteria (blue-green algae). J Phycol 28:737–745
Castenholz RW (2001) General characteristics of the cyanobacteria. In: Boone DR, Castenholz RW (eds) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, pp 474–487
Desikachary TV (1959) Cyanophyta. ICAR Monographs on Algae. Indian Council of Agricultural Research, New Delhi, p 686
Geitler L (1932) Cyanophyceae. In: Rabenhorst L, Osterrich und der Schweiz (eds) Kryptogamen flora van Deutschland, vol XIV. Academische, Leipzig, pp 1–196
Ghelardi E, Celandroni F, Salvetti S, Barsoti C, Baggiani A, Senesi S (2002) Identification and characterization of toxigenic Bacillus cereus isolates responsible for two food-poisoning outbreaks. FEMS Microbiol Lett 208:129–134
Guglielmi G, Rippka R, Tandeau de Marsac N (1993) Main properties that justify the different taxonomic position of Spirulina sp. and Arthrospira sp. among cyanobacteria. In: Doumenge F, Durand-Chastel H, Toulemont A (eds) Spiruline algue de vie. Bulletin de l′Institut Océanographique Monaco, Musée Océanographique, Numéro spécial 12, pp 13–23
Gurtler V, Mayall BC (2001) Genomic approaches to typing, taxonomy and evolution of bacterial isolates. Int J Syst Evol Microbiol 51:3–16
Harrison SP, Mytton LR, Skot L, Dye M, Cresswell A (1992) Characterization of Rhizobium isolates by amplification of DNA polymorphisms using random primers. Can J Microbiol 34:3423–3430
Hertzberg S, Liaaen-Jensen S, Siegelman HW (1971) The carotenoids of blue-green algae. Phytochem 10:3121–3127
Hezazi A, Keane CT, Falkiner FR (1997) The use of RAPD-PCR as a typing method for Serratia marcescens. J Med Microbiol 46:913–919
Hoffmann L, Komarek J, Kastovsky J (2005) System of cyanoprokaryotes (cyanobacteria)—state 2004. Algol Stud 117:95–115
Hu J, van Eysden J, Quiros CF (1995) Generation of DNA-based markers in specific genome regions by two-primers RAPD reaction. PCR Methods Appl 4:346–351
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Jeberlin-Prabina B, Kumar K, Kannaiyan S (2003) Phylogenetic analysis of symbiotic and free living cyanobacterial cultures using DNA amplification fingerprinting. Indian J Exp Biol 41:865–869
Kato T, Watanabe MF, Watanabe M (1991) Allozyme divergence Microcystis (Cyanophyceae) and its taxonomic inference. Arch Hydrobio Suppl 64:129–140
Kaushik BD (1987) Laboratory methods for blue green algae. Associated Publ Co, New Delhi, pp 162
Komarek J (2003) Problem of the taxonomic category “species” in cyanobacteria. Algol Stud 109:281–297
Komarek J, Anagnostidis K (1989) Modern approach to the classification system of Cyanophytes 4—Nostocales. Arch Hydrobiol Suppl 82:247–345
Li R, Denbella HJ, Carmichael WW (2001) Isolates identifiable as Arthrospira maxima and Arthrospira fusiformis (Oscillatoriales, Cyanobacteria) appear identical on the basis of a morphological study in culture and 16S rRNA gene sequences. Phycologia 40:367–371
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lunge VR, Ikuta N, Fonseca ASK, Hirigoyen D, Stoll M, Bonatto S, Ozaki LS (1994) Identification and inter-relationship analysis of Bradyrhizobium japonicum strains by restriction fragment length polymorphism (RFLP) and random amplified polymorphic DNA (RAPD). World J Microbiol Biotechnol 10:648–652
Lyra C, Suomalainen S, Gugger M, Vezie C, Sundman P, Paulin L, Sivonen K (2001) Molecular characterization of planktic cyanobacteria of Anabaena, Aphanizomenon, Microcystis and Planktothrix genera. Int J Syst Evol Microbiol 51:513–526
McGrath A, Higgins DG, Mc Carthy TV (1998) Sequence analysis of DNA randomly amplified from the Saccharomyces cerevisiae genome. Mol Cell Probes 12:397–405
McKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Mishra U, Pabbi S, Dhar DW, Singh PK (2004) Floristic abundance and comparative studies on some specific nitrogen fixing blue green algae isolated from soils of J and K state. Adv Plant Sci 17:635–640
Neilan BA (1995) Identification and phylogenetic analysis of toxigenic cyanobacteria by multiple randomly amplified polymorphic DNA PCR. Appl Environ Microbiol 61:2286–2291
Nishihara H, Miwa H, Watanabe M, Nagashima M, Yagi O, Takamura Y (1997) Random amplified polymorphic DNA (RAPD) analysis for discriminating genotypes of Microcystis cyanobacteria. Biosci Biotechnol Biochem 61:1067–1070
Ordog V, Srirk WA, Lenobel R, Bancirova M, van Staden J, Szigeti J, Nemeth L (2004) Screening microalgae for some potentially useful agricultural and pharmaceutical secondary metabolites. J Appl Phycol 16:309–314
Otsuka S, Suda S, Shibata S, Oyaizu H, Matsumoto S, Watanabe MM (2001) A proposal for the unification of five species of the cyanobacterial genus Microcystis Kutzing ex Lemmermann 1907 under the Rules of the Bacteriological Code. Int J Syst Evol Microbiol 51:873–879
Palinska KA, Liesack W, Rhiel E, Krumbein WE (1996) Phenotype variability of identical genotypes: the need for a combined approach in cyanobacterial taxonomy demonstrated on Merismopedia-like isolates. Arch Microbiol 166:224–233
Peerbooms PG, Engelen MN, Stokman DA, Van Bentham BH, Bruisten SM (2002) Nasopharyngeal carriage of potential bacterial pathogens related to day care attendance, with special reference to the molecular epidemiology of Haemophilus influenzae. J Clin Microbiol 40:2832–2836
Rippka R, Herdman M (1992) Pasteur culture collection of cyanobacteria strains in axenic culture catalogue & taxonomic handbook. Vol I: catalogue of strains. Pasteur Institute, Paris, p 103
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Robinson NJ, Robinson PJ, Gupta A, Bleasby JA, Whitton BA, Morby A (1995) Singular over-representation of an octameric palindrome, HIP1, in DNA from many cyanobacteria. Nucl Acids Res 23:729–773
Rohlf FJ (1995) NYSYS-pc Numerical taxonomy and multivariate analysis system. Version 1.80. Exeter Software. Setauket, New York
Round FE (1981) The ecology of algae. Cambridge University Press, Cambridge, pp 1–653
Shalini, Gupta RK, Dhar DW (2008) Phylogenetic analysis of cyanobacterial strains of genus-Calothrix by single and multiplex randomly amplified polymorphic DNA-PCR. World J Microbiol Biotechnol 24:927–935
Smith JK, Parry JD, Day JG, Smith RJ (1998) A PCR technique based on Hip1 interspersed repetitive sequence distinguishes cyanobacterial species and strains. Microbiology 144:2791–2801
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Stanier RY, Sistrom WR, Hansen TA, Whitton BA, Castenholz RW, Pfennig N, Gorlenko VN, Kondratieva EN, Eimhjellen KE, Whittenbury R, Gherna RL, Truper HG (1978) Proposal to place nomenclature of cyanobacteria (blue-green algae) under rules of international code of nomenclature of bacteria. Int J Syst Bacteriol 28:335–336
Tiwari DN, Mishra AK, Singh AP (2005) Heterocyst differentiation and function in Cyanobacteria. In: Mukherjee et al (eds) Frontiers in plant sciences, pp 757–768
Tomaselli L (1997) Morphology, ultrastructure and taxonomy. In: Vonshak A (ed) Spirulina platensis (Arthrospira): physiology. Cell-biology and biotechnology. Taylor and Francis, London, pp 1–15
Tomaselli L, Palandri MR, Tredici MR (1996) On the correct use of Spirulina designation. Algol Stud 83:539–548
Turner S (1997) Molecular systematics of oxygenic photosynthetic bacteria. Pl Syst Evol (Suppl) 11:13–52
Turpin PJF (1829) Spirulina oscillariode. In: Levrault FG (ed) Dictionnarie des Sciences Naturelles, Paris, pp 309–310
Urbach E, Robertson DL, Chisholm SW (1992) Multiple evolutionary origins of prochlorophytes within the cyanobacterial radiation. Nature 355:267–270
Venkataraman LV, Bhagyalakshmi N, Ravishankar GA (1995) Commercial production of micro and macro algae- problems and potentials. Ind J Microbiol 35:1–19
Virk PS, Ford-Lloyd BV, Jackson MT, Newbury HJ (1995) Use of RAPD for the study of diversity within plant germplasm collections. Heredity 74:170–179
Vonshak A, Tomaselli L (2000) Arthrospira (Spirulina): systematics and ecophysiology. In: Whitton A, Potts M (eds) The ecology of cyanobacteria. Kluwer Academic Publishers, The Netherlands, pp 505–522
Welsh J, McClelland M (1990) Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res 18:7213–7218
Whitton BA, Potts M (2000) Introduction to the cyanobacteria. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht, pp 1–11
Williams JGK, Kubelik AR, Livak KJ, Raflaski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Wilmotte A (1994) Molecular evolution and taxonomy of the cyanobacteria. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, pp 1–25
Wilmotte A, Herdman M (2001) Phylogenetic relationships among the cyanobacteria based on 16S rRNA sequences. In: Boone DR, Castenholz RW (eds) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York, pp 487–493
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Acknowledgments
The authors gratefully acknowledge the facilities provided by the Post Graduate School, Indian Agricultural Research Institute (IARI), New Delhi, India. The first author is also thankful to the Director, Centre for Scientific and Industrial research (CSIR), Govt. of India for extending financial support in the form of Junior Research Fellowship (JRF) and Senior Research Fellowship (SRF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N.K., Dhar, D.W. Phylogenetic relatedness among Spirulina and related cyanobacterial genera. World J Microbiol Biotechnol 27, 941–951 (2011). https://doi.org/10.1007/s11274-010-0537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0537-x