Abstract
Wastewater is increasingly becoming the primary source of potable water in many cities, thanks to the development of recycling facilities. Persistent contaminants such as dyes and perfluorinated compounds from textile industries as well as other contaminants necessitate the design of removal technologies to treat wastewater to reduce these chemicals before discharge or being used as feed to a potable water plant. Several chemical treatment techniques have been reported but the most utilized advanced chemical treatments lead to high costs and further environmental concerns. This study investigated an alternative approach to wastewater treatment using a hydrodynamic cavitation pilot plant combined with a venturi as a way to remove recalcitrant compounds. The optimization of the removal process was explored by testing the effect of orifices with size 2, 3, 4, 5, or 6 mm on the decoloration of orange II dye. The impact of the catalyst: iron(II); oxidizing agent: hydrogen peroxide; and contact time was evaluated to find the ideal conditions under which the removal of perfluorooctanoic acid (PFOA) could be achieved. The decoloration of 20 ppm of orange II dye in simulated industrial textile wastewater was achieved at 90% efficiency when the pressure at the inlet was maintained at 300 kPa, the temperature at 34 °C, the pH at 2, and the orifice size at 2 mm of diameter. The kinetic study proved the decoloration reaction was pseudo first order and the rate of decolourisation of orange II was 0.23/min.Ten parts per million of PFOA could not be degraded by free radical attack using advanced oxidation processes when the inlet pressure was maintained at 300 kPa, the temperature at 34 °C, the pH of 2, and the orifice diameter of 2 mm. This resistance to removal is due to the structure of PFOA which is made up of a fluorine ion which stabilizes the compounds by inductive effects while dye is made up of nitrogen ion and is compatible with the above removal methods. The study demonstrated that the combination of venturi and orifice requires the throat size of the venturi to be similar or equal to that of the orifice for better efficiency.
Similar content being viewed by others
1 Introduction
Safe drinking water is essential to human health and well-being. But unfortunately, nearly two billion people cannot access clean, safe drinking water (Everard, 2019). Safe drinking water is defined as “water with microbial, chemical and physical characteristics that meet WHO guidelines or local standards on drinking water quality” (Herschy, 2012). Consumption of contaminated water, like river water, is responsible for many diseases. The easiest method to purify wastewater is boiling, followed by filtration or vice versa. However, these methods are energy intensive, and the resulting water quality does not necessarily meet potable standards.
Moreover, harmful contaminants often resist boiling temperatures and loss of water through vaporization is another setback. An alternative method is distillation. Though efficient, it possesses the same disadvantages as boiling and filtration, let alone its complexity. Other advanced water purification methods include reverse osmosis, ion exchange, ozonation, and ultraviolet (UV) light. However, these methods require electricity and expensive machinery, thus not appropriate in rural (remote) areas. Coagulation and flocculation are used to aggregate colloids into larger, denser particles that settle more quickly and become more filterable. Several studies have demonstrated the effectiveness of clay coagulants for wastewater treatment (Eikebrokk & Saltnes, 2002; Jiang et al., 2004). However, the potability of water obtained has not reached drinking water standards or demonstrated the necessary quality for dumping in the environment. Coagulation also produces sludges that require safe disposal.
Next to domestic water are industrial effluents which may become a valuable source of potable water if purified adequately. But these effluents are giving rise to environmental concerns that require strict regulations. To narrow the scope of the challenge, a list of priority compounds has been established to decide on the type of action necessary to circumvent water scarcity, water pollution, and poor water quality in cities. Typical pollutants in textile industries are a source of concern. A large amount of industrial effluent contains toxic and bio-refractory organic pollutants such as textiles dyes, aromatics compounds, chlorinated hydrocarbons, and phenolic compounds. Persistent contaminants are chemical compounds that are difficult to remove from water. They resist degradation by chemical, photochemical, or biochemical reactions in water (European Union Policy, 2017 ). Persistent contaminants have a long-life span and stability during transport. In fact, they are semivolatile and can withstand several evaporation cycles, and can be air-lifted so that they can easily be condensed. These compounds undergo bioaccumulation in living tissues. Most persistent compounds are water-soluble; they are toxic at low concentrations to living organisms and the environment (Mustereţ & Teodosiu, 2007). Persistent contaminants induce diseases such as cancer, central nervous problems, reproductive disorders, and disruption of the immune response (Badmus et al., 2018).
The majority of persistent contaminants are generated in chemical industries, including the textile industry, pharmaceutical industry, chemical industry, paper industry, metallurgical industry, mining industry, and petrochemical industry (Mustereţ & Teodosiu, 2007). Wellness and health products have contributed a lot to pollution in water, including hormones, pesticides, and perfluorinated compounds (PFCs), all classed as persistent organic pollutants (POPs). The European Union has drafted standards to regulate and limit the number of persistent contaminants in the environment through the Stockholm agreement by limiting the number of imports or exports of products that present a health hazard to the environment (European Union Policy, 2017). Clothes and textiles are made in industries using many coloring agents and other chemicals, dyes, and perfluorinated compounds. Such compounds have been described as persistent contaminants and are difficult to remove from wastewater using conventional processes.
The side products of textile and chemical manufacturing are discharged into the receiving environment resulting in significant water contamination. These compounds are not completely removed by conventional biological processes (Padhi, 2012). Different methods of treatment have been employed by many researchers to attempt the removal of contaminants that have been unsuccessful. So far, the following techniques have been used on POPs, including carbon bed adsorption, biological methods, oxidation using chlorination and ozonation, electrochemical methods, membrane processes, and other advanced oxidation processes for the removal of organic pollutants over the last few years. It has been reported that some advanced oxidation methods such as photochemical, photocatalytic, ozonation, electrochemical, sonolysis, ionizing radiation, Fenton methods, persulfate oxidation, zero valent iron, enzymatic are capable of removing perfluorinated compounds such as PFOA and PFOS from wastewater (Trojanowicz et al., 2018). Many groups have extensively studied hydrodynamic cavitation (HC) in wastewater treatment during the past decades (Badmus et al., 2020). It has been reported to be energy efficient and easy to upscale to treat industrial effluents. The use of the HC technique to remove persistent organic pollutants (POPs) is applied in textile, pharmaceutical, biofuel synthesis , and food industries (Panda et al., 2020). The HC method achieved positive results in degrading bio-refractory pollutants (Chakinala et al., 2008). Synergistic effects of cavitation with oxidants such as ozone, hydrogen peroxide, Fenton reagents, peroxydisulfate and photooxidation have been reported to eliminate the challenges of separate processes and significantly increase the degradation potential for AOPS (Fedorov et al., 2022). Recently, sulfate radical based advanced oxidation processes is considered as a substitute to traditional AOPS (Fedorov et al., 2020). Oxidants such as persulfate, peroxymonosulfate have been used as a source of sulfate radicals which enhance the performance of hydrodynamic cavitation (Fedorov et al., 2021). Persulfate and peroxymonosulfate can be activated using diverse activators as well as UV, catalyst, heat and base (Cako et al., 2020). Moreover, several advanced rotational hydrodynamic cavitation reactors with potential greater than conventional hydrodynamic reactors have been showed (Sun et al., 2021). Recent trend on application of percarbonate as oxidant in AOPS as an alternative to hydrogen peroxide is considered with several benefit such as wide operating pH range and prevention of acidification (Fedorov et al., 2023).Without disregarding the progress made in the application of HC, studies on the optimization process based on geometric sizes of the orifice plates and venturi are very limited. Hence, the uniqueness of this work was that it uses a single hole orifice studies of various sizes to optimise the system before combining the best orifice with the venturi. It mainly focuses on geometric consideration. This work explored the best ways of removing dyes and perfluorooctanoic acid from the effluents from textile industries using hydrodynamic cavitation, focusing on geometric consideration.
2 Experimental Methods
2.1 Chemicals
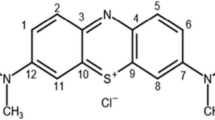
Orange II sodium dye (99% purity, Sigma-Aldrich) was used to simulate dye wastewater. In addition, sulfuric acid (99%, Sigma-Aldrich), hydrogen peroxide (30%, Sigma-Aldrich), ferrous sulfate (99%, Sigma-Aldrich), and perfluorooctanoic acid (96%, Sigma-Aldrich) were all used to complete this study.
2.2 Experimental Setup
Figure 1 depicts a schematic representation of the HC pilot plant and the constriction used in this work. The system mainly consisted of a circular loop made up of a holding tank of 80 L in volume, a centrifugal pump (2650 rpm, 2.2 KW, 400 V), a cavitation chamber involving venturi or orifice, control valves to regulate the flow rate through the constriction, with pressure and temperature gauge devices as indicated.
2.3 Experimental Procedure
The orange II dye solution concentration of 20 ppm was prepared using distilled water. The constant total reaction volume was kept at 10 L. The solution was brought to pH 2 using a small amount of sulfuric acid and placed in the feed tank. The treatment time varied between 10 to 60 min and the sampling interval time was 2 and 10 min, respectively. As reported in a similar investigation, the inlet pressure was adjusted to 300 kPa (Badmus et al., 2020). The flow rate of the sample was optimized by varying cavitating devices (orifices 2, 3, 4, 5, and 6 mm) initially, then using a 10-mm throat for the circular venturi alone (Fig. 2b). The same experiment was repeated with the orifice (Fig. 2a) using 100 mg of iron(II) catalyst in the form of iron sulfate, first and then with 10 mL of an oxidizing agent: hydrogen peroxide (30% purity). The initial experiment was conducted for 60 min with a 10-min sampling time. Then, this experiment was repeated for 10 min without iron as the catalyst or the oxidizing agent. It was this step that defined the optimum cavitation device. The PFOA solution concentration of 10 ppm was prepared using distilled water while the constant total reaction volume was kept at 10 L. The solution was brought to pH 2 using sulfuric acid and placed in the feed tank using a 2-mm orifice alone. The treatment and sampling interval time was 10 and 2 min, respectively. As reported in a similar investigation, the inlet pressure was adjusted to 300 kPa (Badmus et al., 2020).
The percentage decolorization of contaminants was analyzed using either UV-Vis for all or Chemical Oxygen Demand with the DR/2010 spectrophotometer. All the experiments conducted in this work took place at a starting pH 2, temperature of 22 °C as it was observed previously that at an acid pH of 2, the best removal of dyes was observed (Badmus et al., 2020) (Table 1).
2.4 Measurement of Dye and PFOA Concentration
The concentration of orange II in the solution was analyzed using a UV–visible spectrophotometer. A 1-mL sample was taken at each sampling interval and the absorbance was measured at 483 nm, then plotted on a calibration chart obtained by evaluating the absorbance of the standard solution of orange II versus its standard concentrations. The concentration of PFOA in the solution was analyzed using HACH DR/2010 spectrophotometer at an absorbance of 185 nm (Trojanowicz et al., 2018).
3 Results and Discussion
3.1 The Effect of Different Cavitating Devices on Dye Decoloration
Simulated wastewater containing orange II dye was allowed to run for 60 min in the HC pilot plant using orifice 2 (O2), 3 (O3), 4 (O4), 5 (O5), and 6 (O6) mm or the venturi (V) with sampling taking place at 10, 20, 30, 40, 50, and 60 min for each specific orifice respectively or the venturi. Finally, the venturi data were compared to the orifice, as depicted in Fig. 3.
In Fig. 2, orange II dye used as model contaminant showed a rapid decoloration using various orifices. Decoloration was observed between 0 and 10 min for orifice 2 (O2), 3 (O3), and 4 (O4). The venturi (V) showed some increase in decoloration from 10 to 30 min and then decreased in effectiveness from 30 to 60 min, showing an optimum time at around 30 min. Orifice O5 had a good decoloration between 0 and 40 min, while a decline in its effectiveness was observed from 10 to 60 min. Orifice O6 was only effective from 10 to 20 min and then decreased. These observations show that the orifice O2, O3, and O4 obtained the highest decoloration efficiency. Across the 60-min experimental period, it was observed that O2 had a nearly steady state of decoloration; this was not the case with orifices O4 and O5, where in the late stages a decrease was observed for O5 by 60 min and in the case of O4, it showed an increase then a decrease. The reason for this observation lies in the size of the orifice. When the orifice size was larger, the removal was not significant; this is the case too for the venturi, which had a throat with a size of 10 mm. A large throat or orifice size or constriction results in a lower fluid flow rate, thus less pressure build-up; in these conditions, literature reports that an excessive pressure recovery would result in the formation of larger cavities that do not collapse, hence reducing the number of hydroxyl radicals generated and lowering the percentage decoloration of orange II dye at the same time. This showed that decoloration could best be achieved under specific condition of pressure and temperature (Saharan et al., 2012). Gągol et al. and Gogate suggest a temperature rise reduces the decoloration rate (Gągol et al., 2018; Gogate, 2007). Temperature increased with time, from 22, 25, 27, 30, 32, and 34 °C at 0, 2, 4, 6, 8, and 10 min respectively.
3.2 The Effect of Orifice Size Coupled with Iron Sulfate or/and Hydrogen Peroxide
3.2.1 Removal of Dye Using Iron Sulfate
The parameters applied remained at 20-ppm concentration for orange II dye, a 10-L volume, 100 mg iron sulfate, pH 2 solution, 60-min experiment, sampling interval of 10 min, 300 kPa of inlet pressure, and 22 °C starting temperature.
Introducing iron(II) into the solution resulted in a different outcome (Fig. 4). The venturi did not provide good decoloration due to the size of the throat as discussed above, i.e., being large and the iron(II) did not result in any improvement. The decoloration of orange II in Fig. 4 showed the impact of iron sulfate addition on dye decoloration. Adding a catalyst in the process of dye decoloration improved the outcome. As observed before, the initial increase of dye decoloration was more significant in the region between 0 and 10 min. In fact, when comparing Figs. 3 and 4, orifice 2 showed an increase from 82 to 93% after 10 min when iron sulfate was added. Likewise, orifice 3 decoloration efficiency increased from 90 to 93%, and orifice 4 showed an increase from 70 to 84%. However, orifices 5 and 6 caused the system efficiency to decrease after 10 min in the presence of the catalyst. Orifice 6 efficiency decreased from 85 to 75%, and orifice 5 decreased from 65 to 44%. This decrease can be attributed to orifice size being too large causing a depletion of hydroxyl radicals resulting in lower oxidation of dye (Chakinala et al., 2008).
3.2.2 Removal of Dye When Adding 10 mL Hydrogen Peroxide
Figure 5 shows dye decoloration upon adding 10 mL of 10 ppm of hydrogen peroxide, an oxidizing agent. The mechanism of H2O2 activation includes the decomposition of H2O2 in perhydroxyl anions which are responsible of oxidation capability of H2O2 (Fedorov et al., 2022). The degradation takes place via radical pathway which led to the production of trace amount of 4-aminobenzen sulfonic acid, aniline, cyclohexa-2,5-diene-1,4-dione and naphthalene (Badmus et al., 2020). The presence of inorganics anions such as NO3-, SO42-,and Cl- in natural water might affect the effectiveness of hydrodynamic cavitation (Fedorov et al., 2023). Water matrix plays a major role on the generation of nitration processes. The nitrogen fixation reactions result in the generation of variation of reactive nitrogen species which play a prominent role on the transformation of organic pollutants during wastewater treatment. It has been reported that nitrated by-products are more toxic than the primary pollutants (Rayaroth et al., 2022). However, the process proved efficient for all the orifices used except for the 6 mm one, where a drastic decrease in decoloration was noticeable. In fact, decoloration was rapid within 10 min for all orifices. When comparing the decoloration between Figs. 3 and 5 at 10 min, orifice 2 increased from 70 to 87%. The percentage decoloration of orange II dye. For instance, at 10 min, the percentage decoloration of orange II dye was slightly reduced for orifices 2, 3, 4, and 5, respectively, from 82 to 77%, from 90 to 86%, from 83 to 82%, and from 65 to 40%. After 10 min, the decoloration continued with a smaller gradient so that decoloration increased slightly. This decrease can be assigned to the lower hydrogen peroxide concentration (molar ratio of 1:30). It was also reported that the non-reacted H2O2 increases the COD. However, the COD of the non-reacted H2O2 is lower than the COD of the contaminants existing in the sample before treatment though the interference is not quickly evident (Groele & Foster, 2019). At the molar ratio of 1:30 of hydrogen peroxide with respect to dye, only slight enhancement of orange II dye degradation was observed because of the lower hydrogen peroxide concentration (Gore et al, 2014). Although the percentage of decoloration decreased, the removal trends indicated an efficient process but additional H2O2 should be carefully dosed (Saharan et al., 2011).
The effect of orifice size in the presence of hydrogen peroxide on dye decoloration (fixed parameters: orange II concentration 20 ppm, solution volume 10 L, hydrogen peroxide 10 mL, solution pH 2, an experiment run for 60 min, sampling interval of 10 min, the inlet pressure of 300 kPa, starting temperature 22 °C)
3.2.3 Removal of Orange II Using Fenton Reagents
The use of 100 mg of iron sulfate mixed with 10 mL of 10 ppm hydrogen peroxide to form a solution known as the Fenton reagent was applied to the decoloration processes using the various orifices. The physical parameters such as temperature, pressure, feedstock volume, pH, and initial temperature remained the same as in the initial experiment. No venturi data were collected due to the size of the throat.
The decoloration process in Fig. 6 showed the effect of Fenton reagents (iron and hydrogen peroxide) on the decoloration. The combined effect of the oxidizing agent and the catalyst was predicted in theory to improve the decoloration process (Alves et al., 2019; Rajoriya et al., 2016; Saharan et al., 2011). However, the outcome of this reaction showed otherwise, although the efficiency of dye removal kept a good trend. While the orifices (O2) , (O3), and (O4) showed good decoloration, orifice (O6) was inefficient due to its large orifice size. Based on the comparison between Figs. 3 and 6, it was recorded at 10 min that in the case of the orifices 2, 3, 4, and 6 mm, the percentage decoloration decreased respectively from 82 to 79%, 90 to 89%, 83 to 78%, and 65 to 39%. This decrease can be attributed to an excessive amount of Fenton reagents causing a poor generation of hydroxyl radical, decreasing the percentage decoloration of orange II (Saharan et al., 2011). Slow decoloration can be assigned to the low generation rate of OH radicals due to the slow reaction of complex iron, which may be generated at pH > 4 in water, or this can be due to the scavenging effect of hydrogen peroxide by excess protons (Badmus et al., 2018).
The effect of decoloration using orifice sizes 2, 3, 4, 5, and 6 in the presence of iron sulfate and hydrogen peroxide. Orange II concentration, 20 ppm; solution volume, 10 L; iron sulfate, 100 mg; hydrogen peroxide, 10 mL; solution pH, 2; experiment time, 60 min. The sampling interval of 10 min, the inlet pressure of 300 kPa, and starting temperature of 22 °C
We have noted that most degradation was efficient below 10 min; therefore, the system should be optimized in that time frame for the orifices alone since the venturi produced almost no meaningful results, being of too low concentration due to throat size.
3.3 Optimization of the Decoloration Process
3.3.1 Removal of Orange II Using Various Orifices and the venturi
Dye decoloration below 10 min using various orifices (2, 3, 4, 5, and 6 mm) was recorded. The same conditions were maintained apart from the time, which was reduced to 10 min and more frequent sampling interval, which was shortened to 2 min apart. The venturi system was tested for comparison.
In Fig. 7, dye decoloration with the various orifice sizes was achieved below 10 min without the addition of peroxide or iron(II). The highest percentage decoloration of orange II dye (90%) at 10 min was achieved for orifice 2 compared to orifice 3, 4, 5, and 6 mm and compared to the venturi, which had 88, 87, 85, 83, and 57% decoloration rates. This can be attributed to the fact that a small-diameter single hole generates more cavitation intensity than a large-diameter single hole as reported in table 2. As the single orifice diameter increased from 2, 3, 4, 5 to 6 mm, the orange II dye decoloration percentage decreased from 90, 88, 87, 85 to 83%, respectively. However, the difference between these percentages of decoloration was not significant. This could be due to the slight difference in orifice single-hole diameter size. The venturi decoloration rate below 10 min showed a positive decoloration. The venturi throat was 10 mm, making the venturi perform much more poorly than the 6-mm hole in decoloration. Other size constrictions for the venturi might improve its performance.
The impact of different cavitating devices (orifice and venturi) on dye decoloration in the HC pilot plant (fixed parameters: orange II concentration 20 ppm, solution volume 10 L, solution pH 2, experiment run for 10 min, sampling interval of 2 min, the inlet pressure of 300 kPa, starting temperature 22 °C)
3.3.2 Observed Hydrodynamic Cavitation Under Optimized Conditions
To optimize, we run the experiment for 10 min with a sampling interval time of 2 min. The experiment was conducted for the 2-mm orifice (O2) alone, the venturi alone (V), and the combination of the orifice and the venturi (O2V) to evaluate the three modes of dye decoloration of 10 L of 20-ppm dye with neither hydrogen peroxide nor iron(II) at pH 2, inlet pressure of 300 kPa, and starting temperature of 22 °C.
The decoloration of orange II dye presented in Fig. 8 was a comparative study of the orifice, venturi, with the combination of both orifice and venturi. Orifice 2 mm achieved the highest percentage of decoloration on its own than in combination with the venturi. In a similar experiment, Gągol et al. (2018) found that the orifice with the smallest hole diameter provided a maximum rate of removal of triglycerides in a similar set up (Gągol et al., 2018). This can be assigned to many generated cavities. In this case, the cavitation event limited the mass transfer resistance while improving collapse (Lalwani et al., 2020). The venturi had the disadvantage of having a fixed throat size five times that of the selected orifice. It would thus not be expected to be better than the orifice decoloration. Nevertheless, the combination of the two provided a trend reinforcing the observation that the combination of both techniques yielded better results than the process with the venturi alone; thus, there may be synergy.
The effect of an orifice, a venturi, and the combination of both cavitating devices on dye decoloration in the HC pilot plant (fixed parameters: orifice 2-mm hole, orange II concentration 20 ppm, solution volume 10 L, solution pH 2, experiment run for 10 min, sampling interval of 2 min, the inlet pressure of 300 kPa, starting temperature 22 °C)
Chemical Kinetics
The determination of the order of reaction for the degradation of orange II dye solution is important. Reactant A represents 20 ppm orange II dye solution. The order of reaction was found using an integration method based on the optimum conditions results which were obtained using orifice 2 mm (O2), the venturi (V), and the combination of orifice 2 mm and the venturi (O2V). Figures 9, 10, and 11 represent the plot of the zero-, first-, and second-order reaction at the optimum conditions.
First-order rate reaction plot for removal of orange II using optimized conditions (fixed parameters: orifice 2 mm, orange II concentration 20 ppm, solution volume 10 L, solution pH 2, experiment run for 10 min, sampling interval of 2 min, inlet pressure of 300 kPa, starting temperature 22 °C). With CAO being the initial concentration of orange II and CA concentration at a given time t
The plots of orifice 2 mm (O2), venturi (V), and the combination of orifice 2 mm and venturi (O2V) showed respectively that the reaction is second order with the highest regression coefficient R2 and the slope equal to 0.966 and 0.0395 L/mg min respectively (orifice 2-mm diameter); 0.9266 and 0.0102 L/mg min (venturi); and 0.9731 and 0.0059 L/mg min (orifice 2-mm diameter and the venturi). However, due to no change in the concentration in water, the reaction has a tendency to respond like a first order with regression coefficient R2 and the slope respectively equal to 0.9228 and 0.23 min−1 using orifice 2-mm diameter; 0.9075 and 0.1087 min−1 using venturi; and 0.9223 and 0.0808 min−1 using the combination of both orifice 2-mm diameter and the venturi. Therefore, the reaction is pseudo-first-order, the rate of decolorization of Orange II (0.23/min) is directly proportional to the concentration of orange II \(-r= k [C_{\mathrm{OR}2}]= 0.23\, \mathrm{min} ^{-1} [C_{\mathrm{OR2}}]\) (using orifice 2 mm). C stands for the concentration of dye in mole/l, k represents the rate constant (min−1), and t is the time in minutes.
Badmus (2019) and Wang et al. (2009) confirmed in their work that dye decolorization with HC pilot plant followed a pseudo-first-order law. Badmus’s experimental run employed two orifices in an hour-long experiment on the HC pilot plant. Both the two orifices and venturi had a 4-mm diameter and were aligned parallel to each other. Wang et al. (2019) made use of a jet loop cavitation device for 1.5 h to reach the same conclusion as Badmus (2019) and Wang et al. (2009).
4 Recommendation
The measurement of COD is suggested to be included in future work that will take this work further. For the purpose of this work, the focus was turned toward the extraction of data only.
4.1 Removal of Perfluorooctanoic Acid Using Optimum Conditions
The optimum experimental condition found for dye degradation under 10 min was used to degrade PFOA with a sampling time of 2 min. The optimum condition was applied to remove perfluorooctanoic acid, namely 300-kPa pressure at the inlet, initial concentration of 10 ppm, pH 2 solution, initial temperature of 22 °C, and orifice 2 mm (Fig. 12). The percentage removal was measured by the amount of chemical oxygen demand using Hach DR/2010 spectrophotometer at absorbance of 185 nm.
The result of monitoring the chemical oxygen demand (COD) demonstrated that the initial concentration at time zero of PFOA was 18 mg/L. As time passed, the COD showed an increased COD value, while the successful removal required the COD content to decrease significantly (Fig.12). This resistance to removal is attributed to the structure of the PFOA which is made up of fluorine ions, which stabilized the compounds by inductive effects while the dye which is made of nitrogen bond was compatible with the above-discussed methods of removal of dyes. For the successful removal of COD, Vitez et al. recommended 70 mg/L as a minimum efficiency value for removing PFOA (Vítez et al., 2012). In our case, the removal of PFOA was not successful which highlights that these PFAS molecules are intractable to hydroxide radical attack. Despite the fact that some molecules are more likely to radical attachment than others, we made the intriguing discovery that PFAS molecules are resistant to the attachment of hydrogen peroxide radicals. This was the case despite the fact that some molecules are more likely to radical attachment than others. In spite of the fact that some molecules have a higher propensity than others for radical attachment, this was nevertheless the case.
5 Conclusion
We investigated the efficiency of a single orifice hole of various diameters in removing the wastewater persistent contaminants by hydrodynamic cavitation. On the one hand, the orifice with a 2-mm hole diameter was found to be ideal to achieve 90% decoloration of orange II dye within 10 min compared to the efficiency of orifices 3, 4, 5, and 6 mm, which yielded 88, 87, 85, and 83% respectively between 0 and 10 min under the applied conditions. On the other hand, the efficiency of the 10-mm throat venturi on the removal of orange II dye was evaluated and yielded a mere 57% decoloration after 10 min of operation. This observation was attributed to the fact that the 10-mm venturi diameter throat is too large compared to the tested orifices; hence, the venturi generated low cavitation intensity due to low-pressure recovery and low flow rate at the 10-mm constriction. The combined 2-mm orifice hole and the 10-mm venturi were assessed, yielding a 71% decoloration efficiency. However, the efficiency of a combined orifice of 2 mm and the venturi is lower than when using an orifice of 2 mm alone. Finally, various operating parameters such as contact time, catalyst, oxidizing agent, and Fenton reagents were investigated. The results showed that a high decoloration of orange II occurred between 0 and 10 min for all experiments, but the results were not significantly higher than the orifice by itself which shows chemical addition is not needed. After 10 min, the decoloration percentage was changing with a small gradient so that the run using the Fenton reagent had a more significant gradient compared to the experiment with none of the elements. Chemical kinetics were evaluated and the rate flow was 0.23/min. The order of the reaction was pseudo first order. The dye removal percentage (90% orange II decoloration) by HC system using orifice 2 mm hole within 10 min time falls within the range of dye removal percentage using physical, biological and chemical processes which are 86.8-99%, 76-90.1% and 88.9-99 % respectively (Katheresan et al., 2018). Hydrodynamic cavitation proves to be energy efficient for the decoloration of orange II dye with a percentage efficiency which was found to be 38%. While dye response to the technique was successful, it was observed that PFOA remains recalcitrant to removal and thus offers more rooms for research on the topic.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Alves, P. H. L., Silva, P. S. L. de, Ferreira, D. C., & Gonçalves, J. C. S. I. de. (2019). COD removal from sucrose solution using hydrodynamic cavitation and hydrogen peroxide: A comparison between Venturi device and orifice plate. Revista Brasileira de Recursos Hidricos, 24, 1–8. https://doi.org/10.1590/2318-0331.241920180147
Badmus, K. O. (2019). Treatment of persistent organic pollutants in wastewater with combined advanced oxidation. University of the Western Cape.
Badmus, K. O., Tijani, J. O., Massima, E., & Petrik, L. (2018). Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Environmental Science and Pollution Research, 25, 7299–7314. https://doi.org/10.1007/s11356-017-1171-z
Badmus, K. O., Irakoze, N., Adeniyi, O. R., & Petrik, L. (2020). Synergistic advance Fenton oxidation and hydrodynamic cavitation treatment of persistent organic dyes in textile wastewater. Journal of Environmental Chemical Engineering, 8, 103521. https://doi.org/10.1016/j.jece.2019.103521
Cako, E., Gunasekaran, K. D., Cheshmeh Soltani, R. D., & Boczkaj, G. (2020). Ultrafast degradation of brilliant cresyl blue under hydrodynamic cavitation based advanced oxidation processes (AOPs). Water Resources and Industry, 24, 100134. https://doi.org/10.1016/j.wri.2020.100134
Chakinala, A. G., Gogate, P. R., Burgess, A. E., & Bremner, D. H. (2008). Treatment of industrial wastewater effluents using hydrodynamic cavitation and the advanced Fenton process. Ultrasonics Sonochemistry, 15, 49–54. https://doi.org/10.1016/j.ultsonch.2007.01.003
Eikebrokk, B., & Saltnes, T. (2002). NOM removal from drinking water by chitosan coagulation and filtration through lightweight expanded clay aggregate filters. Journal of Water Supply: Research and Technology - AQUA, 51, 323–332. https://doi.org/10.2166/aqua.2002.0029
European Union Policy, E .(2017). Science for environment policy future brief : Persistent organic pollutants : Towards a POPs-free future.
Everard, M. (2019). Meeting global drinking water needs. Nature Sustainability, 2, 360–361. https://doi.org/10.1038/s41893-019-0292-4
Fedorov, K., Plata-Gryl, M., Khan, J. A., & Boczkaj, G. (2020). Ultrasound-assisted heterogeneous activation of persulfate and peroxymonosulfate by asphaltenes for the degradation of BTEX in water. Journal of Hazardous Materials, 397, 122804. https://doi.org/10.1016/j.jhazmat.2020.122804
Fedorov, K., Sun, X., & Boczkaj, G. (2021). Combination of hydrodynamic cavitation and sr-aops for simultaneous degradation of BTEX in water. Chemical Engineering Journal, 417, 128081. https://doi.org/10.1016/j.cej.2020.128081
Fedorov, K., Dinesh, K., Sun, X., Soltani, R. D. C., Wang, Z., Sonawane, S., & Boczkaj, G. (2022). Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon – A review. Chemical Engineering Journal, 432, 134191. https://doi.org/10.1016/j.cej.2021.134191
Fedorov, K., Rayaroth, M. P., Shah, N. S., & Boczkaj, G. (2023). Activated sodium percarbonate-ozone (SPC/O3) hybrid hydrodynamic cavitation system for advanced oxidation processes (AOPs) of 1,4-dioxane in water. Chemical Engineering Journal, 456, 141027. https://doi.org/10.1016/j.cej.2022.141027
Gągol, M., Przyjazny, A., & Boczkaj, G. (2018). Wastewater treatment by means of advanced oxidation processes based on cavitation – A review. Chemical Engineering Journal, 338, 599–627. https://doi.org/10.1016/j.cej.2018.01.049
Gogate, P. R. (2007). Application of cavitational reactors for water disinfection: Current status and path forward. Journal of Environmental Management, 85, 801–815. https://doi.org/10.1016/j.jenvman.2007.07.001
Gore, M. M., Saharan, V. K., Pinjari, D. V., Chavan, P. V., & Pandit, A. B. (2014). Degradation of reactive orange 4 dye using hydrodynamic cavitation based hybrid techniques. Ultrasonics Sonochemistry, 21, 1075–1082. https://doi.org/10.1016/j.ultsonch.2013.11.015
Groele, J., & Foster, J. (2019). Hydrogen peroxide interference in chemical oxygen demand assessments of plasma treated waters. Plasma, 2, 294–302. https://doi.org/10.3390/plasma2030021
Herschy, R. W. (2012). Water quality for drinking: WHO guidelines. Encycl Earth Sci Ser, 876–883. https://doi.org/10.1007/978-1-4020-4410-6_184
Jiang, J. Q., Zeng, Z., & Pearce, P. (2004). Preparation and use of modified clay coagulants for wastewater treatment. Water, Air, & Soil Pollution, 158, 53–65. https://doi.org/10.1023/B:WATE.0000044833.75579.8b
Katheresan, V., Kansedo, J., & Lau, S. Y. (2018). Efficiency of various recent wastewater dye removal methods: A review. Journal of Environmental Chemical Engineering, 6, 4676–4697. https://doi.org/10.1016/j.jece.2018.06.060
Lalwani, J., Gupta, A., Thatikonda, S., & Subrahmanyam, C. (2020). Oxidative treatment of crude pharmaceutical industry effluent by hydrodynamic cavitation. Journal of Environmental Chemical Engineering, 8. https://doi.org/10.1016/j.jece.2020.104281
Mustereţ, C. P., & Teodosiu, C. (2007). Removal of persistent organic pollutants from textile wastewater by membrane processes. Environmental Engineering and Management Journal, 6, 175–187. https://doi.org/10.30638/eemj.2007.022
Padhi, B. (2012). Pollution due to synthetic dyes toxicity & carcinogenicity studies and remediation. International Journal of Environmental Sciences, 3, 940–955. https://doi.org/10.6088/ijes.2012030133002
Panda, D., Saharan, V. K., & Manickam, S. (2020). Controlled hydrodynamic cavitation: A review of recent advances and perspectives for greener processing. Processes, 8. https://doi.org/10.3390/pr8020220
Rajoriya, S., Carpenter, J., Saharan, V. K., & Pandit, A. B. (2016). Hydrodynamic cavitation: An advanced oxidation process for the degradation of bio-refractory pollutants. Reviews in Chemical Engineering, 32, 379–411. https://doi.org/10.1515/revce-2015-0075
Rayaroth, M. P., Aravindakumar, C. T., Shah, N. S., & Boczkaj, G. (2022). Advanced oxidation processes (AOPs) based wastewater treatment - Unexpected nitration side reactions - A serious environmental issue: a review. Chemical Engineering Journal, 430, 133002. https://doi.org/10.1016/j.cej.2021.133002
Saharan, V. K., Badve, M. P., & Pandit, A. B. (2011). Degradation of Reactive Red 120 dye using hydrodynamic cavitation. Chemical Engineering Journal, 178, 100–107. https://doi.org/10.1016/j.cej.2011.10.018
Saharan, V. K., Pandit, A. B., Satish Kumar, P. S., & Anandan, S. (2012). Hydrodynamic cavitation as an advanced oxidation technique for the degradation of acid red 88 dye. Industrial and Engineering Chemistry Research, 51, 1981–1989. https://doi.org/10.1021/ie200249k
Sun, X., You, W., Xuan, X., Ji, L., Xu, X., Wang, G., Zhao, S., Boczkaj, G., Yoon, J. Y., & Chen, S. (2021). Effect of the cavitation generation unit structure on the performance of an advanced hydrodynamic cavitation reactor for process intensifications. Chemical Engineering Journal, 412. https://doi.org/10.1016/j.cej.2021.128600
Trojanowicz, M., Bojanowska-Czajka, A., Bartosiewicz, I., & Kulisa, K. (2018). Advanced oxidation/reduction processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – A review of recent advances. Chemical Engineering Journal, 336, 170–199. https://doi.org/10.1016/j.cej.2017.10.153
Vítez, T., Ševcíková, J., & Oppeltová, P. (2012). Evaluation of the efficiency of selected wastewater treatment plant. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis, 60, 173–180. https://doi.org/10.11118/actaun201260010173
Wang, X.-K., Zhang, S.-Y., & Li, S.-P. (2009). Decolorization of reactive brilliant red K-2BP in aqueous solution by using hydrodynamic cavitation. Environmental Engineering Science, 26. https://doi.org/10.1089/ees.2007.0201
Funding
Open access funding provided by Cape Peninsula University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kakama, N.K., Petrik, L.F. & Ojumu, T.V. The Optimization of Hydrodynamic Cavitation as an Advanced Oxidation Option for the Removal of Persistent Contaminants in Wastewater. Water Air Soil Pollut 235, 193 (2024). https://doi.org/10.1007/s11270-024-06924-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-06924-w