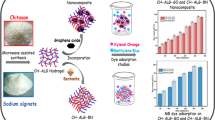
Abstract
A bentonite clay-based hetero-metallic oxide composite, i.e., KAB-Ben, was synthesized by impregnation of bentonite clay with potassium salt of Al-substituted Keggin tungstoborate (KAB) via a simple co-precipitation method. The composite was morphologically characterized by FTIR, SEM-EDX, XRD, and TGA, and its efficiency for removal of rhodamine B (RhB) from wastewater was investigated to compare with unmodified bentonite. The adsorptive potential was explored by changing the experimental parameters such as contact time, adsorbent dose, pH, temperature, agitation speed, and the reusability. KAB-Ben composite was able to remove 92.2% of RhB within the first 20 min which was much higher than bentonite. The qmax values of bentonite and KAB-Ben were 14.2 and 208.3 mg/g, respectively. Kinetic study revealed that the adsorption mechanism followed pseudo-second-order kinetics. Furthermore, the adsorbent was able to be reused thrice without any drastic loss of adsorption activity. The composite possessed satisfactory tolerance over various ranges of pH, temperature, and dye concentration. The current research is an insight into a new cost-effective and environmentally benign adsorbent for detoxification of RhB from aqueous solution.
Graphical Abstract











Similar content being viewed by others
Data Availability
All data is presented in the “Results” section along with references.
Abbreviations
- EDX:
-
Energy dispersive X-ray
- FTIR:
-
Fourier transform infrared
- KAB:
-
Potassium salt of Al-substituted Keggin tungstoborate
- Mnt:
-
Montmorillonite
- Pzc:
-
Point of zero charge
- SEM:
-
Scanning electron microscopy
- TGA:
-
Thermogravimetric analysis
- TEM:
-
Transmission electro-microscopy
- XRD:
-
X-ray powder diffraction
- UV:
-
Ultraviolet
References
Abdolrahimi, N., & Tadjarodi, A. (2019). Adsorption of rhodamine-B from aqueous solution by activated carbon from almond shell. Multidisciplinary Digital Publishing Institute Proceedings, 41(1), 51.
Achmad, A., Kassim, J., Suan, T. K., Amat, R. C., & Seey, T. L. (2012). Equilibrium, kinetic and thermodynamic studies on the adsorption of direct dye onto a novel green adsorbent developed from Uncaria gambir extract. Journal of Physical Science, 23(1), 1–13.
Al-Maliky, E. A., Gzar, H. A., & Al-Azawy, M. G. (2021). Determination of point of zero charge (PZC) of concrete particles adsorbents. In IOP Conference Series: Materials Science and Engineering (Vol. 1184, No. 1, p. 012004). IOP Publishing.
Alcântara, R. R., Muniz, R. O. R., & Fungaro, D. A. (2016). Full factorial experimental design analysis of rhodamine B removal from water using organozeolite from coal bottom ash. International Journal of Energy and Environment, 7(5), 357–374.
Ali, M., & Sreekrishnan, T. R. (2001). Aquatic toxicity from pulp and paper mill effluents: A review. Advances in Environmental Research, 5(2), 175–196.
Alkan, M., Çelikçapa, S., Demirbaş, Ö., & Doğan, M. (2005). Removal of reactive blue 221 and acid blue 62 anionic dyes from aqueous solutions by sepiolite. Dyes and Pigments, 65(3), 251–259.
Alkan, M., Demirbaş, Ö., Çelikçapa, S., & Doğan, M. (2004). Sorption of acid red 57 from aqueous solution onto sepiolite. Journal of Hazardous Materials, 116(1-2), 135–145.
Attique, S., Batool, M., Yaqub, M., Goerke, O., Gregory, D. H., & Shah, A. T. (2020a). Highly efficient catalytic pyrolysis of polyethylene waste to derive fuel products by novel polyoxometalate/kaolin composites. Waste Management & Research, 38(6), 689–695.
Attique, S., Batool, M., Yaqub, M., Gregory, D. H., Wilson, C., Goerke, O., & Shah, A. T. (2020b). Synthesis and catalytic performance of cesium and potassium salts of aluminum substituted tungstoborate for pyrolysis of polyethylene waste to petrochemical feedstock. Materials Chemistry and Physics, 246, 122781.
Ayawei, N., Godwin, J., & Wankasi, D. (2015). Synthesis and sorption studies of the degradation of Congo red by Ni-Fe layered double hydroxide. International Journal of Applied Chemical Sciences Research, 13(3), 1197–1217.
Baidya, K. S., & Kumar, U. (2021). Adsorption of brilliant green dye from aqueous solution onto chemically modified areca nut husk. South African Journal of Chemical Engineering, 35, 33–43.
Banat, I. M., Nigam, P., Singh, D., & Marchant, R. (1996). Microbial decolorization of textile-dyecontaining effluents: A review. Bioresource Technology, 58(3), 217–227.
Barakat, Mohamed Abou Elfetouh, Kumar, Rajeev, Seliem, Moaaz Korany, Selim, Ali Qurany, Mobarak, Mohamed, Anastopoulos, Ioannis, . . . Mohamed, Essam Abdelrahman. (2020). Exfoliated clay decorated with magnetic iron nanoparticles for crystal violet adsorption: Modeling and physicochemical interpretation. Nanomaterials, 10(8), 1454.
Basharat, S., Rehman, R., Mahmud, T., Basharat, S., & Mitu, L. (2020). Tartaric acid-modified Holarrhena antidysenterica and Citrullus colocynthis biowaste for efficient eradication of crystal violet dye from water. Journal of Chemistry, 2020, 1–18.
Basir, N. M., Lintang, H. O., & Endud, S. (2015). Phosphotungstic acid supported on acid-leached porous kaolin for Friedel-crafts acylation of anisole. Jurnal Teknologi, 76(13), 27–34.
Bhattacharyya, K. G., SenGupta, S., & Sarma, G. K. (2014). Interactions of the dye, rhodamine B with kaolinite and montmorillonite in water. Applied Clay Science, 99, 7–17.
Brown, G. M., Noe-Spirlet, M.-R., Busing, W. R., & Levy, H. A. (1977). Dodecatungstophosphoric acid hexahydrate, (H5O2+) 3 (PW12O403−). The true structure of Keggin 'spentahydrate' from single-crystal X-ray and neutron diffraction data. Acta Crystallographica Section B: Structural Crystallography and Crystal. Chemistry, 33(4), 1038–1046.
Chawla, S., Uppal, H., Yadav, M., Bahadur, N., & Singh, N. (2017). Zinc peroxide nanomaterial as an adsorbent for removal of Congo red dye from waste water. Ecotoxicology and Environmental Safety, 135, 68–74.
Chen, Q., Tian, Y., Li, P., Yan, C., Pang, Y., Zheng, L., Deng, H., Zhou, W., & Meng, X. (2017). Study on shale adsorption equation based on monolayer adsorption, multilayer adsorption, and capillary condensation. Journal of Chemistry, 2017(12), 1–11.
Cheung, C. W., Porter, J. F., & Mckay, G. (2001). Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Research, 35(3), 605–612.
Damiyine, B., Guenbour, A., & Boussen, R. (2017). Rhodamine B adsorption on natural and modified Moroccan clay with cetyltrimethylammonium bromide: Kinetics, equilibrium and thermodynamics. Journal of Materials and Environmental Science, 8(3), 860–871.
Das, S. K., Bhowal, J., Das, A. R., & Guha, A. K. (2006). Adsorption behavior of rhodamine B on Rhizopus oryzae biomass. Langmuir, 22(17), 7265–7272.
Deshpande, A. V., & Kumar, U. (2002). Effect of method of preparation on photophysical properties of Rh-B impregnated sol–gel hosts. Journal of Non-Crystalline Solids, 306(2), 149–159.
Dong, B.-B., Zhang, B.-B., Wu, H.-Y., Chen, X., Zhang, K., & Zheng, X.-C. (2013). Synthesis, characterization and catalytic evaluation of SBA-15 supported 12-tungstophosphoric acid mesoporous materials in the oxidation of benzaldehyde to benzoic acid. Materials Research Bulletin, 48(7), 2491–2496.
Elmorsi, R. R., Abou-El-Sherbini, K. S., El-Dein, S., Waleed, A., & Lotfy, H. R. (2022). Activated eco-waste of Posidonia oceanica rhizome as a potential adsorbent of methylene blue from saline water. Biomass Conversion and Biorefinery, 1–14.
Eniola, J. O., Kumar, R., Al-Rashdi, A. A., Ansari, M. O., & Barakat, M. A. (2019). Fabrication of novel Al (OH) 3/CuMnAl-layered double hydroxide for detoxification of organic contaminants from aqueous solution. ACS Omega, 4(19), 18268–18278.
Ferreira, B. C. S., Teodoro, F. S., Mageste, A. B., Gil, L. F., de Freitas, R. P., & Gurgel, L. V. A. (2015). Application of a new carboxylate-functionalized sugarcane bagasse for adsorptive removal of crystal violet from aqueous solution: Kinetic, equilibrium and thermodynamic studies. Industrial Crops and Products, 65, 521–534.
Foo, K. Y., & Hameed, B. H. (2010). Decontamination of textile wastewater via TiO2/activated carbon composite materials. Advances in Colloid and Interface Science, 159(2), 130–143.
Fujii, T., Ishii, A., & Anpo, M. (1990). Absorption and fluorescence spectra of rhodamine B molecules encapsulated in silica gel networks and their thermal stability. Journal of Photochemistry and Photobiology A: Chemistry, 54(2), 231–237.
Gaballah, I., & Kilbertus, G. (1998). Recovery of heavy metal ions through decontamination of synthetic solutions and industrial effluents using modified barks. Journal of Geochemical Exploration, 62(1-3), 241–286.
Gamelas, J. A. F., Soares, M. R., Ferreira, A., & Cavaleiro, A. M. V. (2003). Polymorphism in tetra-butylammonium salts of Keggin-type polyoxotungstates. Inorganica Chimica Acta, 342, 16–22.
Gao, L.-H., Wang, Y.-B., & Bai, L.-J. (2011). Synthesis, spectroscopy and photocatalytic activity of B elements substituted tungstoborate heteropoly complexes. Spectroscopy and Spectral Analysis, 31(8), 2191–2194.
Ghibate, R., Senhaji, O., & Taouil, R. (2021). Kinetic and thermodynamic approaches on rhodamine B adsorption onto pomegranate peel. Case Studies in Chemical and Environmental Engineering, 3, 100078.
Giri, Balendu Shekher, Gun, Sudeshna, Pandey, Saurabh, Trivedi, Aparna, Kapoor, Riti Thapar, Singh, Rajendra Prasad, . . . Chaturvedi, Preeti. (2020). Reusability of brilliant green dye contaminated wastewater using corncob biochar and Brevibacillus parabrevis: Hybrid treatment and kinetic studies. Bioengineered, 11(1), 743-758.
Gong, R., Li, M., Yang, C., Sun, Y., & Chen, J. (2005). Removal of cationic dyes from aqueous solution by adsorption on peanut hull. Journal of Hazardous Materials, 121(1-3), 247–250.
Guo, Y., Zhao, J., Zhang, H., Yang, S., Qi, J., Wang, Z., & Xu, H. (2005). Use of rice husk-based porous carbon for adsorption of rhodamine B from aqueous solutions. Dyes and Pigments, 66(2), 123–128.
Habeeb, O. M. A. R. A. B. E. D., Ramesh, K., Ali, G. A. M., Yunus, R. M., & Olalere, O. A. (2017). Kinetic, isotherm and equilibrium study of adsorption capacity of hydrogen sulfide-wastewater system using modified eggshells. IIUM Engineering Journal, 18(1), 13–25.
Hacıosmanoğlu, G. G., Mejías, C., Martín, J., Santos, J. L., Aparicio, I., & Alonso, E. (2022). Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review. Journal of Environmental Management, 317, 115397.
Han, H., Rafiq, M. K., Zhou, T., Xu, R., Mašek, O., & Li, X. (2019). A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. Journal of Hazardous Materials, 369, 780–796.
Hebbar, R. S., Isloor, A. M., Prabhu, B., Asiri, A. M., & Ismail, A. F. (2018). Removal of metal ions and humic acids through polyetherimide membrane with grafted bentonite clay. Scientific Reports, 8(1), 4665.
Hema, M, & Arivoli, S. (2009). Rhodamine B adsorption by activated carbon: Kinetic and equilibrium studies. Indian Journal of Chemical Technology, 16(1), 38–45.
Hill, C. L. (1998). Introduction: Polyoxometalates multicomponent molecular vehicles to probe fundamental issues and practical problems. Chemical Reviews, 98(1), 1–2.
Ho, Y.-S., & McKay, G. (1998). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70(2), 115–124.
Ho, Y.-S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465.
Ianoş, R., Păcurariu, C., Muntean, S. G., Muntean, E., Nistor, M. A., & Nižňanský, D. (2018). Combustion synthesis of iron oxide/carbon nanocomposites, efficient adsorbents for anionic and cationic dyes removal from wastewaters. Journal of Alloys and Compounds, 741, 1235–1246.
Ibrahim, M. B., Haruna, M. A., & Ibrahim, A. M. (2012). Optimization of crystal violet dye removal from aqueous solution using agro wastes. ChemSearch Journal, 3(1), 28–33.
Ighalo, J. O., & Adeniyi, A. G. (2020). Adsorption of pollutants by plant bark derived adsorbents: An empirical review. Journal of Water Process Engineering, 35, 101228.
Jha, A. K. (2016). Bentonite minerals and its TGA, DSC and PXRD studies. Journal of the Indian Chemical Society, 93(4), 437–442.
Jönsson, B., Åkesson, T., Jönsson, B., Meehdi, S., Janiak, J., & Wallenberg, R. (2009). Structure and forces in bentonite MX-80 (No. SKB-TR--09-06). Swedish Nuclear Fuel and Waste Management Co..
Kabbashi, N. A., Atieh, M. A., Al-Mamun, A., Mirghami, M. E. S., Alam, M. D. Z., & Yahya, N. (2009). Kinetic adsorption of application of carbon nanotubes for Pb (II) removal from aqueous solution. Journal of Environmental Sciences, 21(4), 539–544.
Kakali, G., Perraki, T. H., Tsivilis, S., & Badogiannis, E. (2001). Thermal treatment of kaolin: The effect of mineralogy on the pozzolanic activity. Applied Clay Science, 20(1-2), 73–80.
Kausar, A., Iqbal, M., Javed, A., Aftab, K., Bhatti, H. N., & Nouren, S. (2018). Dyes adsorption using clay and modified clay: A review. Journal of Molecular Liquids, 256, 395–407.
Konicki, W., Siber, D., & Narkiewicz, U. (2017). Removal of rhodamine B from aqueous solution by ZnFe2O4 nanocomposite with magnetic separation performance. Polish Journal of Chemical Technology, 19(4), 65–74.
Kowanga, K. D., Gatebe, E., Mauti, G. O., & Mauti, E. M. (2016). Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu (II) and Pb (II) using defatted Moringa oleifera seed powder. The journal of phytopharmacology, 5(2), 71–78.
Leodopoulos, C., Doulia, D., & Gimouhopoulos, K. (2015). Adsorption of cationic dyes onto bentonite. Separation and Purification Reviews, 44(1), 74–107.
Li, F., Chen, Y., Huang, H., Cao, W., & Li, T. (2015). Removal of rhodamine B and Cr (VI) from aqueous solutions by a polyoxometalate adsorbent. Chemical Engineering Research and Design, 100, 192–202.
Li, Y., Yan, X., Hu, X., Feng, R., & Zhou, M. (2019). Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of rhodamine B in water. Chemical Engineering Journal, 375, 122003.
Li, Z., Potter, N., Rasmussen, J., Weng, J., & Lv, G. (2018). Removal of rhodamine 6G with different types of clay minerals. Chemosphere, 202, 127–135.
Liu, K., Li, H., Wang, Y., Gou, X., & Duan, Y. (2015). Adsorption and removal of rhodamine B from aqueous solution by tannic acid functionalized graphene. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 477, 35–41.
Liu, Y., & Liu, Y.-J. (2008). Biosorption isotherms, kinetics and thermodynamics. Separation and Purification Technology, 61(3), 229–242.
Mahmoodi, N. M., & Arami, M. (2009). Degradation and toxicity reduction of textile wastewater using immobilized titania nanophotocatalysis. Journal of Photochemistry and Photobiology B: Biology, 94(1), 20–24.
Mao, X., Zhang, J.-N., Gao, L.-H., Su, Y., Chen, P.-X., & Wang, K.-Z. (2016). An electrostatically self-assembled thin film made of Zn-substituted tungstoborate and rhodamine B with photoelectrochemical properties. Journal of Nanoscience and Nanotechnology, 16(4), 3674–3678.
McKay, G. (1982). Adsorption of dyestuffs from aqueous solutions with activated carbon I: Equilibrium and batch contact-time studies. Journal of Chemical Technology and Biotechnology, 32(7-12), 759–772.
Mittal, A., Mittal, J., Malviya, A., & Gupta, V. K. (2010). Removal and recovery of chrysoidine Y from aqueous solutions by waste materials. Journal of Colloid and Interface Science, 344(2), 497–507.
Moussout, H., Ahlafi, H., Aazza, M., & Maghat, H. (2018). Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala International Journal of Modern Science, 4(2), 244–254.
Ouachtak, H., Haouti, E., Rachid, E. G., Anouar, H., Redouane, A., Elhassan, A., Addi, A. A., Akbal, F., & Taha, M. L. (2020). Experimental and molecular dynamics simulation study on the adsorption of rhodamine B dye on magnetic montmorillonite composite γ-Fe2O3@Mt. Journal of Molecular Liquids, 309, 113142.
Ouellet-Plamondon, C. M., Stasiak, J., & Al-Tabbaa, A. (2014). The effect of cationic, non-ionic and amphiphilic surfactants on the intercalation of bentonite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 444, 330–337.
Özacar, M., & Şengil, İ. A. (2005). Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresource Technology, 96(7), 791–795.
Pandey, N., Kamboj, N., Bharti, M., Kamboj, V., Sharma, S., & Bisht, A. (2020). An overview on enormous effect of hazardous wastes on water components and their management. Advances in Environmental Pollution Management: Wastewater Impacts and Treatment Technologies, 1, 158–173.
Patil, S. P., Bethi, B., Sonawane, G. H., Shrivastava, V. S., & Sonawane, S. (2016). Efficient adsorption and photocatalytic degradation of rhodamine B dye over Bi2O3-bentonite nanocomposites: A kinetic study. Journal of Industrial and Engineering Chemistry, 34, 356–363.
Pettignano, A., Tanchoux, N., Cacciaguerra, T., Vincent, T., Bernardi, L., Guibal, E., & Quignard, F. (2017). Sodium and acidic alginate foams with hierarchical porosity: Preparation, characterization and efficiency as a dye adsorbent. Carbohydrate Polymers, 178, 78–85.
Pokhrel, D., & Viraraghavan, T. (2004). Treatment of pulp and paper mill wastewater—A review. Science of the Total Environment, 333(1-3), 37–58.
Postai, D. L., Demarchi, C. A., Zanatta, F., Melo, D. C. C., & Rodrigues, C. A. (2016). Adsorption of rhodamine B and methylene blue dyes using waste of seeds of Aleurites moluccana, a low cost adsorbent. Alexandria Engineering Journal, 55(2), 1713–1723.
Raha, S., Ivanov, I., Quazi, N. H., & Bhattacharya, S. N. (2009). Photo-stability of rhodamine-B/montmorillonite nanopigments in polypropylene matrix. Applied Clay Science, 42(3-4), 661–666.
Koohi, S. R., Allahyari, S., Kahforooshan, D., Rahemi, N., & Tasbihi, M. (2019). Natural minerals as support of silicotungstic acid for photocatalytic degradation of methylene blue in wastewater. Journal of Inorganic and Organometallic Polymers and Materials, 29, 365–377.
Ravindra Reddy, T., Kaneko, S., Endo, T., & Lakshmi Reddy, S. (2017). Spectroscopic characterization of bentonite. J. Lasers Opt. Photonics, 4, 1–4.
Raza, A., Rehman, R., & Batool, M. (2022). Recent review of Titania-clay-based composites emerging as advanced adsorbents and photocatalysts for degradation of dyes over the last decade. Adsorption Science & Technology, 2022(1), 1–15
Rehman, R., Raza, A., Yasmeen, F., Dar, A., Al-thagafi, Z. T., & Meraf, Z. (2022). Recent literature review of significance of polypyrrole and its biocomposites in adsorption of dyes from aqueous solution. Adsorption Science & Technology, 2022, 1–18.
dos Santos, R., de Oliveira Bruno, H. C., & Melgar, L. Z. (2019). Use of bentonite calcined clay as an adsorbent: Equilibrium and thermodynamic study of rhodamine B adsorption in aqueous solution. Environmental Science and Pollution Research, 26(28), 28622–28632.
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77(3), 247–255.
Rocchiccioli-Deltcheff, C., Fournier, M., Franck, R., & Thouvenot, R. (1983). Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum (VI) and tungsten (VI) compounds related to the Keggin structure. Inorganic Chemistry, 22(2), 207–216.
Saha, P., & Chowdhury, S. (2011). Insight into adsorption thermodynamics. Thermodynamics, 16, 349–364.
Salleh, M. A., Mohd, M., Khalid, D., Karim, W. A., Abdul, W., & Idris, A. (2011). Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination, 280(1-3), 1–13.
Santos, I. C. M. S., Simões, M. M. Q., Pereira, M. M. M. S., Martins, R. R. L., Neves, M. G. P. M. S., Cavaleiro, J. A. S., & Cavaleiro, A. M. V. (2003). Oxidation of monoterpenes with hydrogen peroxide catalysed by Keggin-type tungstoborates. Journal of Molecular Catalysis A: Chemical, 195(1-2), 253–262.
Selvam, P. P., Preethi, S., Basakaralingam, P., Thinakaran, N., Sivasamy, A., & Sivanesan, S. (2008). Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. Journal of Hazardous Materials, 155(1-2), 39–44.
Shukla, A., Zhang, Y.-H., Dubey, P., Margrave, J. L., & Shukla, S. S. (2002). The role of sawdust in the removal of unwanted materials from water. Journal of Hazardous Materials, 95(1-2), 137–152.
Tézé, A., Michelon, M., & Hervé, G. (1997). Syntheses and structures of the tungstoborate anions. Inorganic Chemistry, 36(4), 505–509.
Thilagan, J., Gopalakrishnan, S., & Kannadasan, T. (2013). Thermodynamic study on adsorption of copper (II) ions in aqueous solution by chitosan blended with cellulose & cross linked by formaldehyde, chitosan immobilised on red soil, chitosan reinforced by banana stem fibre. International Journal of Scientific Research Engineering & Technology, 2(1), 28–36.
Thompson, G., Swain, J., Kay, M., & Forster, C. F. (2001). The treatment of pulp and paper mill effluent: A review. Bioresource Technology, 77(3), 275–286.
Vega-Negron, A. L., Alamo-Nole, L., Perales-Perez, O., Gonzalez-Mederos, A. M., Jusino-Olivencia, C., & Roman-Velazquez, F. R. (2018). Simultaneous adsorption of cationic and anionic dyes by chitosan/cellulose beads for wastewaters treatment. International Journal of Environmental Research, 12, 59–65.
Verma, V. K., & Mishra, A. K. (2010). Kinetic and isotherm modeling of adsorption of dyes onto rice husk carbon. Global NEST Journal, 12(2), 190–196.
Vijayakumar, G., Tamilarasan, R., & Dharmendirakumar, M. (2012). Adsorption, kinetic, equilibrium and thermodynamic studies on the removal of basic dye rhodamine-B from aqueous solution by the use of natural adsorbent perlite. Journal of Materials and Environmental Science, 3(1), 157–170.
Wang, S., Boyjoo, Y., & Choueib, A. (2005). A comparative study of dye removal using fly ash treated by different methods. Chemosphere, 60(10), 1401–1407.
Wang, S., Yang, B., & Liu, Y. (2017). Synthesis of a hierarchical SnS2 nanostructure for efficient adsorption of Rhodamine B dye. Journal of Colloid and Interface Science, 507, 225–233.
Wekoye, J. N., Wanyonyi, W. C., Wangila, P. T., & Tonui, M. K. (2020). Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environmental Chemistry and Ecotoxicology, 2, 24–31.
Wirawan, T., Koesnarpadi, S., & Widodo, N. T. (2020). Study of rhodamine B adsorption onto activated carbon from spent coffee grounds. In American Institute of Physics Conference Series (Vol. 2237, No. 1, p. 020007).
Xing, S., Bing, Q., Song, L., Li, G., Liu, J., Shi, Z., Feng, S., & Xu, R. (2016). The uncommon channel-based Ln-MOFs for highly selective Fe3+ detection and superior rhodamine B adsorption. Chemistry–A. European Journal, 22(45), 16230–16235.
Yahia, I. S., Rammah, Y. S., & Khaled, K. F. (2013). Fabrication of an electrochemical cell based on rhodamine B dye for low power applications. Journal of Materials and Environmental Science, 4(3), 442–447.
Doan, Y., Hai, T., Chu, M., Phuong, T., Dinh, T. D., Nguyen, T. H., Vo, T., Cam, T., Nguyen, N. M., et al. (2020). Adsorptive removal of rhodamine B using novel adsorbent-based surfactant-modified alpha alumina nanoparticles. Journal of Analytical Methods in Chemistry, 2020, 6676320.
Yusuf, T. L., Orimolade, B. O., Masekela, D., Mamba, B., & Mabuba, N. (2022). The application of photoelectrocatalysis in the degradation of rhodamine B in aqueous solutions: A review. RSC Advances, 12(40), 26176–26191.
Zeng, B., Li, J., Xiong, C., Lin, G., Wang, W., & Wu, Z. (2022). High-performance Zn-based coordination polymers selectively adsorb mercury ions from aqueous solutions. Journal of Cleaner Production, 337, 130551.
Zhang, H., Zhao, R., Liu, Z., Zhang, X., & Du, C. (2023). Enhanced adsorption properties of polyoxometalates/coal gangue composite: The key role of kaolinite-rich coal gangue. Applied Clay Science, 231, 106730.
Zhang, J., Hu, X., Yan, X., Feng, R., Zhou, M., & Xue, J. (2019). Enhanced adsorption of rhodamine B by magnetic nitrogen-doped porous carbon prepared from bimetallic ZIFs. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 575, 10–17.
Zhu, W., Liu, J., & Li, M. (2014). Fundamental studies of novel zwitterionic hybrid membranes: Kinetic model and mechanism insights into strontium removal. The Scientific World Journal, 2014, 485820.
Acknowledgements
The authors are grateful to the Researchers Supporting Project number (RSP2023R407), King Saud University, Riyadh, Saudi Arabia, for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raza, A., Rehman, R., Batool, M. et al. Adsorptive Elimination of Rhodamine B Dye by Synthetic Clay-Based Hetero-metallic Oxide Nanocomposite KAB-Ben for Rapid Wastewater Treatment. Water Air Soil Pollut 234, 654 (2023). https://doi.org/10.1007/s11270-023-06589-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06589-x