Abstract
Anaerobic digestates from biogas plants can be used as agricultural fertilisers providing recycling nitrogen (N) and other nutrients for crop needs. It is still unclear the impact on phosphorus (P) losses to runoff waters of digestates as sources of N instead of inorganic N fertilisers in over fertilised soils with P. A field experiment was done in a sandy and acidic soil high in P. The experimental design was completely randomised with five treatments. The inorganic N fertilisation (90 kg ha−1) was done in four treatments, those with past P inputs of the following: (i) inorganic N and P fertilisers (Ni/MF), (ii) organic amendments (pig or duck dry slurry-Ni/PS and Ni/DS or cattle manure compost-Ni/CM). Digestate was applied in plots with past P input of cattle slurry (DG/CS) providing also 90 kg N ha−1. Ryegrass was sowed as cover crop. The concentration of total dissolved P in runoff waters was high in all treatments and ranged between 0.5 (Ni/PS) and 2.6 mg L−1 (DG/CS). These runoff waters pose a risk of non-source P pollution for fresh waters. In soils with low P sorption capacity and over fertilised with P, the fertilisation with anaerobic digestate as the only source of N to crops increased the risk of P losses to runoff waters compared with inorganic N fertilisation. Therefore, the amount of digestate applied to soil must be calculated considering its N:P ratio in order to not exceed the crop P requirement.
Similar content being viewed by others
1 Introduction
Soils over fertilised with phosphorus (P) are a potential risk for the eutrophication of fresh waters through non-point source pollution from agricultural areas. The use of P surplus beyond crop needs has been occurring not only by an inadequate inorganic P fertilisation but also by the application of organic amendments to soil. Organic amendments like manures and composts have in general low N:P ratios as they are often enriched with P relative to N concerning plant needs (Kleinman & Sharpley, 2003) resulting its continuous agricultural use in P over fertilisation (Eghball, 2002). This P accumulated in the soils is called legacy P and can be lost via erosion, runoff and leaching decreasing the efficiency of P use. Soil legacy P accumulates in forms of decreasing bioavailability through time due to its high affinity for sorption and occlusion in particles of the soil solid phase like low crystallinity oxides of Al and Fe (Frossard et al., 1995; Torrent, 1997; Horta and Torrent, 2007a), edges of silicate clays and calcite (Matar et al., 1992) and organic complexes of Al and Fe (Borggaard et al., 2004; Gerke & Hermann, 1992). Manage soil legacy P to overcome its environmental risk can be achieved by stopping the inorganic P fertilisation. This strategy considers that the content of soil P is enough to crop needs and its uptake will decrease the soil P through time. MacDowell et al. (2020) in a work at a Countryside scale in New Zealand refer that decrease soil test P (STP) to an agronomic target (Olsen P of 20.0 mg kg−1) by halting the P fertilisation can be easier and achieved in less time than to meet the requirements of the environmental target (water extractable P — WEP of 0.02 mg L−1). These authors referred that the time to reach the agronomic target was up to 11.8 years and to the environmental target was 26–55 years. The level of available P in soils evaluated by a STP like Olsen P and the soil sorption capacity are main factors that influence the losses of P from soils to runoff waters. An Olsen P value of 20 mg kg−1 can be adopted as a conservative threshold value for agricultural and also environmental purposes in sandy and acidic soils of the temperate zone (Horta and Torrent, 2007b).
However, nitrogen fertilisation is still necessary to maintain crop production. Currently, the target of the bioeconomy is to improve the sustainability of the resources by the implementation of processes leading to the use of renewable biomass. In agriculture, this target means for example lowering the use of inorganic fertilisers and increasing the use of biowastes like animal manures, slurries or crop residues (Eur-Lex, 2015; European Commission, 2018). In addition, these biowastes can be used as feedstock to an anaerobic digestion process with the biogas production (mainly CH4) and a final by-product called digestate. Biogas can replace fossil fuel energy at the farm level and the digestate shows fertilising value, namely for N crop fertilisation (Tambone et al., 2009; Tani et al., 2006). In addition, digestate also contains P ranging between 2 and 35 g kg−1 DM and contains organic matter ranging between 550 and 680 g kg−1 DM (Teglia et al., 2011). It is still unclear how digestate can influence P availability in soils and consequently P losses to runoff waters. A field experiment was done in order to get a deep knowledge about the impact on P losses from soil to runoff waters by halting P fertilisation in a soil high in P. In addition, the N fertilisation was done with digestate or with inorganic fertiliser in order to evaluate the effect of these two fertilisers on P losses from soil to runoff waters. The experiment was done in an acidic sandy soil high in P in plots with previous application of inorganic P fertiliser or different animal manures using ryegrass as cover crop.
So, the main objectives of the present work were as follows:
-
Assessment of P losses to runoff waters from a soil over fertilised with P after halting P fertilisation;
-
To compare P losses to runoff waters between treatments fertilised with inorganic N or with digestate as source of N in field plots over fertilised with P and subject in the past to fertilisation with different biowastes.
2 Materials and Methods
2.1 Site Description and Experimental Design
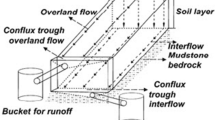
The field study was conducted at an experimental erosion station located on a farm belonging to the Polytechnic Institute of Castelo Branco (39° 49′ 25.278″ N; − 7° 26′ 48.603″ W) during 2018–2019. This field consisted of 15 plots of 42 m2 (22.1-m length and 1.9-m width) with a 9% slope, each one equipped with a device installed at the bottom to collect superficial runoff waters and sediments. This device consisted of two reservoirs connected through a pipe with a flow divider. When runoff was too high and the first box full, 1/9 of the water surplus entered the second box. The soil underlying all plots is a Dystric Regosol (IUSS, 2015) developed on granitic rocks. These soils are mainly used for rain-fed pastures and for cereal crops such as rye and barley. The region has a sub humid Mediterranean climate, with a mean annual temperature of 15.4 °C and mean annual rainfall of 738 mm.
The experiment of this work was conducted in the same field and plots that were used in 2012–13 and 2013–2014 for the assessment of phosphorus (P) losses to runoff waters after soil P fertilisation with different organic amendments and an inorganic fertiliser as described by Horta et al. (2018). Briefly that experiment consists on a completely randomised design with 4 treatments each one with 3 replicates making a total of 12 plots. The 4 treatments considered were as follows: triple superphosphate (MF) as the reference P fertilisation; cattle manure compost (CM); solid fraction of pig slurry (PS); solid fraction of duck slurry (DS). This trial was carried out during two crop seasons between October 2012 and May 2014 and the crop was an annual ryegrass. In addition, three plots were fertilised with cattle slurry (CS) in 2012–2013 and in 2013–2014 were left fallow. In 2014–2015 and 2015–2016 crop seasons, all these plots were sowed with annual ryegrass and fertilised only with inorganic N in order to evaluate the availability to crops of the soil legacy P. In 2016–2017 and 2017–2018 crop seasons, the plots were left fallow. In 2018–2019 (the period of the present work), a treatment with the use of a solid digestate (DG) as a source of nitrogen to the annual rye grass was included in the CS plots. To all the other plots of the field experiment, the fertilisation was done only with inorganic N (Ni). So, in 2018–2019, the field experiment consisted of a completely randomised experimental design, with 5 treatments each one with 3 replicates making a total of 15 plots. The 5 treatments considered were as follows: inorganic N fertilisation as the reference N fertilisation Ni/MF (in the same plots with 2012–2014 application of inorganic P and N fertilisation-MF); inorganic N fertilisation Ni/DS (in the same plots with 2012–2014 application of the solid fraction of duck slurry-DS); inorganic N fertilisation Ni/PS (in the same plots with 2012–2014 application of the solid fraction of pig slurry-PS); inorganic N fertilisation Ni/CM (in the same plots with 2012–2014 application of cattle manure compost-CM); digestate application DG/CS (in the plots with cattle slurry application-CS in 2012–2013). The trial was carried out during one crop season from 3 December 2018 (rye grass sowing) to 20 May 2019 (rye grass harvest). Two weeks before sowing, in November 2018, digestate was incorporated into soil at an amount equal to the application of 90 kg N ha−1. The same N rate was used for the inorganic N fertilisation (90 kg N ha−1), but in these treatments, it was divided into three applications: 35 kg N ha−1 at sowing in the form of ammonium sulphate, and two topdressing applications with 35 kg N ha−1 in March and 20 kg N ha−1 in April, both in the form of an ammonium-nitrate fertiliser (Table 1). As soil was high in potassium (average ≈ 106 mg K kg−1 by the ammonium lactate method; Egnér et al., 1960), no K was added during the trial. According to the objectives of the field experiment, no P fertilisation was done.
Annual ryegrass (cv. winter star II) was sowed in all the experimental area. Lolium sp. was chosen since it is a cover crop that can be used in integrated agricultural systems to provide P accumulated in their biomass after decomposition to subsequent crops reducing the demand of inorganic P fertilisers (Soltangheisi et al., 2020) and is also the main forage crop in temperate regions (Humphreys, 2005).
2.2 Analysis
2.2.1 Digestate
The digestate used in this work was the same used by Horta and Carneiro (2020). It was obtained at a biogas plant from a mixture of cattle slurry and rye straw during 30 days of retention time in mesophilic reactors with 3000 m3 of capacity. Then, the digestate slurry was pressed to separate the solid and the liquid phase. In this work we used the solid phase of the digestate.
The methods used to quantify the moisture content, pH (digestate to water ratio of 1:5), electric conductivity (1:10 suspension of digestate to water ratio), organic matter total N, Ca and Mg were described at Horta and Carneiro (2020). Total P (PT) was determined after ignition at 550 °C during 3 h and extracted with H2SO4 0.5 M, inorganic P (Pi) was extracted with H2SO4 0.5 M and then quantified by molecular absorption spectrophotometry. Organic P (Po) was calculated as the difference between PT and Pi. P forms were evaluated by the sequential fractionation scheme of Traoré et al. (1999), using a digestate to solution ratio of 1:200 (wt/vol). The first extraction was performed with H2O (H2O-P), the second with 0.5 M NaHCO3 (pH 8.5; NaHCO3-P), the third with 0.1 M NaOH (NaOH-P) and the fourth with 1 M HCl (HCl-P). The inorganic P forms (Pi) in water or in the bicarbonate extracts are considered easily available to crops; the Pi extracted in NaOH solution is mainly bound to Fe and Al oxides or metal–organic complexes, and thus considered moderately labile; the Pi extracted in HCl is bound mainly to Ca in low-solubility precipitates, such as apatite or octacalcium phosphate, and thus considered as stable P forms (Traoré et al., 1999; Gagnon et al., 2012). Total dissolved P in the extracts of the first three fractions was analysed after acid potassium persulphate digestion (American Public Health Association, 2012) and the dissolved organic P (Po) was calculated as the difference between the total dissolved P and inorganic P quantified in each fraction. Water soluble P (WSP) was quantified after extraction at a 1:250 (digestate to water ratio). WSP is considered a reliable mean to predict dissolved reactive P concentration in runoff waters from surface-applied manures and biosolids (Elliot et al., 2005). All of the P extracts were centrifugated at 3000 rpm and P in the supernatant was quantified by the method of Murphy and Riley (1962).
Total cations such as Fe, Mn, Cu, Pb, Cd, Ni and Cr were also extracted by the aqua regia digestion and quantified by atomic absorption spectrophotometry.
The chemical composition of the solid digestate is presented in Table 2. The standards of some European countries (AFNOR, 2001; Siebert, 2008; Decree-Law 103/2015) specifies for products to be considered as soil amendments (those that have a beneficial effect on soil OM) should have an OM content between 20 and 90% in a DM basis and that they should have a dry matter content higher than 30–50% (AFNOR, 2006; Decree-Law 103/2015). The C/N ratio should be higher than 8 (AFNOR, 2006). So, overall the digestate meet these standards except for the dry matter content slightly below the minimum threshold level (29%). Also, the electric conductivity (EC of 1.7 dS m−1) of the digestate was within the normal range to prevent soil salinity considering that 3 dS m−1 is the threshold value (Ayers & Westcott, 1994). Regarding the N content, DG had 85% of the total N in organic forms, and this fact together with a C:N ratio of 12 suggests that the rate of N mineralisation of DG can be slow. Total P content of the digestate (11.7 g kg−1) was within the range (2–35 g kg−1) referred by other works (Möller et al., 2010; Teglia et al., 2011; Voća et al., 2005). Digestate has 98% of its total P in inorganic P forms. The sum of the P forms evaluated by the sequential fractionation scheme was 11.6 g kg−1 with also 98% in inorganic forms. Easily available forms account for 60% of the total Pi forms (39 and 21% for Pi-H2O and Pi-NaHCO3 respectively) and 40% for forms of medium (Pi-NaOH 5%) and of lower availability (Pi-HCl 35%). The P fraction that is water soluble, i.e. WSP/PT was 23%. This value is lower than those referred by Brandt et al. (2004) for triple superphosphate (85%) and for dairy manure (52%), similar to that for poultry manure (21%) and lower than for biosolids (2.5%). The WSP of DG (2.7 g kg−1) was lower than that referred by García-Albacete et al. (2014) for digestate obtained from municipal solid wastes (3.6 g kg−1). The levels of other nutrients in DG such as Cu, Zn Fe, Mn and Ni and non-nutrients such as Cd, Cr and Pb (data not shown) were below the threshold levels compared with those established for agricultural use of composts by the Portuguese legislation (Decree-Law 103/2015).
Concerning the microbiological analysis, the digestate was free of microorganisms of faecal origin such as Escherichia coli which had 6.3 × 102 CFU g−1 and Salmonella spp. which was not detected. These values comply with the above-mentioned legislation the Decree-Law 103/2015. Overall, the digestate used in this work meets the quality requirement criteria defined by the European Union (JRC, 2014) for its land application.
2.2.2 Soil
Prior to the installation of the experiment in December 2018 and at the end of the experiment in May 2019, composite soil samples were taken from each plot from 0 to 0.20 m of depth. Soil samples were air dried, sieved (< 2 mm) and analysed for pH (H2O), electric conductivity (EC), organic matter (OM), textural class and Olsen P. The pH was measured using a pH electrode by taking 10.0 g of dried and sieved soil and 25 mL of distilled water (1:2.5, soil to solution ratio) which were in contact for 1 h. The EC was measured with a conductivimeter at a suspension of a soil to water ratio of 1:2 after 60-min shaking. The organic matter was analysed using a potentiometric titration method according to the procedure described in Walkley and Black (1934). The soil texture was evaluated by the particle size analysis using the pipet method (Deshpande & Telang, 1950). Crop available potassium was quantified by the method of Egnér et al. (1960). The Olsen P (Olsen et al., 1954) was used to evaluate the soil available P not only to the crops but also to the transfer from soil to runoff waters (Horta and Torrent, 2007b). Soil P sorption capacity was evaluated by the Fox and Kamprath (1970) procedure and fitted to Langmuir equation in order to estimate the Qmax (maximum sorption P capacity of the soil). The orthophosphate P in solution was determined by the molybdate blue method of Murphy and Riley (1962).
The soil of the experiment was sandy loam. At the beginning of the experiment (December 2018), the soils of each plot were acidic with a pH value (mean value of the 3 repetitions for each treatment) ranged between 5.1 in Ni/MF and 6.4 Ni/PS and DG/CS. The EC was low with values between 0.09 dS m−1 in Ni/MF and 0.14 dS m−1 in Ni/DS. The soils had a medium level of organic matter ranging between 2.8% in Ni/DS and 3.1% in Ni/CM and a high level of available K ranging between 82 in Ni/MF and 159 mg K kg−1 in Ni/CM. All plots were over fertilised with P with high level of Olsen P ranging between 31 mg P kg−1 in Ni/MF and 87 mg P kg−1 in Ni/DS (INIAP, 2006; Horta et al., 2010). The maximum P sorption capacity of the soil was low (Qmax = 95 mg P kg−1).
2.2.3 Surface Runoff Waters
During the 2018–2019 crop season, ten samples of runoff water occurring under natural rainfall were taken from the collection devices located at the downslope end of each plot. The height of water in each device after each runoff event was measured to calculate the volume of runoff (L ha−1). Runoff water samples were centrifuged with an acceleration of about ≈ 9000 m s–2 for 10 min prior to filtration (< 0.45 µm) to determine Dissolved reactive P (DRP0.45) and Total dissolved P (TDP). Total dissolved P (TDP) was analysed after acid persulphate digestion during 1 h in autoclave at 120 °C. Dissolved organic P (DOP) was calculated as the difference between TDP and DRP. Water samples were also centrifuged at ≈ 1.04 × 105 m s–2 for 15 min and P soluble and in particles ø < 0.023 µm was quantified (DRP0.023).
Orthophosphate P in solution was determined by the molybdate blue method of Murphey and Riley (1962).
2.3 Statistical Analyses
Statistical analysis of the data was carried out with IBM SPSS statistics 26 software (www.spss.com). The data were analysed by linear regression, Pearson’s correlation coefficients and analysis of variance ANOVA. For the P content in the runoff waters and for the soil properties, the model of ANOVA analysis used was the one-way ANOVA with five treatments. Duncan’s test was used to compare means at 0.05 probability level.
3 Results
3.1 Surface Runoff Waters and Sediments
In the 2018–2019 crop season, from December 3rd, 2018, to May 20th, 2019, the rainfall was low (212 mm) compared with the climatological normal of 1986–2015 for the same time period (433 mm from December to May). Consequently, not only the number but also the amount of water from the runoff events was low. Nevertheless, during the crop season, it was possible to sample water from ten runoff water events. The rainfall during the crop season produced a total amount of runoff water of 412 L ha−1 in DG/CS, 504 L ha−1 in Ni/MF, 513 L ha−1 in Ni/CM, 888 L ha−1 in Ni/PS and 1155 L ha−1 in Ni/DS.
The loss of the sediments after the rainfall events was vestigial and thus the quantification of particulate P losses by erosion was not possible.
The average pH values of the runoff water during the crop cycle were in the neutral range between 7.0 ± 0.08 in Ni/DS and 7.2 ± 0.15 in Ni/MF treatments, with no significant differences between the treatments.
The average electric conductivity had no significant differences between the treatments and ranging from 0.08 ± 0.02 in Ni/PS to 0.18 ± 0.06 dS m−1 in Ni/MF. The low EC of runoff water observed in all the treatments is in accordance with the also low EC in the soil of each treatment (< 0.4 dS m−1; INIAP, 2006) and also with the relatively low values of EC in the digestate (1.7 dS m−1). The soil EC values ranged between 0.09 ± 0.01 dS m−1 in DG/CS and 0.25 ± 0.06 in Ni/CM at the end of the experiment (crop cycle). The EC values observed in the runoff waters were low concerning the water quality for irrigation (EC < 0.7 dS m−1 water without restrictions for crop irrigation; Ayers and Westcot, 1994) and even for potable water (EC < 2.5 dS m−1; Decree-Law nº306/2007).
3.2 P Losses in Surface Runoff Waters
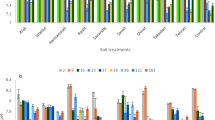
The average values of inorganic P (DRP0.45) in the runoff waters were 0.43 mg L−1 in Ni/PS, 0.85 in Ni/MF, 0.95 in Ni/CM, 0.97 in Ni/DS and 1.99 in DG/CS.
The DRP0.45 was significantly correlated with DRP0.023 by the following regression equation:
The DRP of the DG/CS treatment was significantly (P < 0.05) higher (1.99 mg L−1) than the DRP of Ni/PS (0.43 mg L−1) and had no significant differences from the other treatments (0.85 to 0.97 mg L−1, Fig. 1a).
Dissolved P in runoff waters: (a) dissolved reactive phosphorus (DRP0.45 and DRP0.023; mean ± SE); capital letters indicate statistical differences between the treatments for DRP0.45 and small letters for DRP0.023. (b) Total dissolved phosphorus (TDP = DOP0.45 and DRP0.45; mean ± SE); capital letters indicate statistical differences between the treatments for TDP and small letters for DOP0.45. Different letters above the columns indicate significant differences between the treatments by the Duncan test at the P < 0.05 level
Losses of P were mainly in inorganic forms (DRP0.45 > DOP0.45; Fig. 1b). However, the digestate application resulted in a significant increase (P < 0.05) in the level of organic P of the runoff waters (DOP0.45 = 0.7 mg L−1) compared to the other treatments (0.06 mg L−1 in Ni/DS and Ni/PS – 0.1 mg L−1 in Ni/MF and Ni/CM). The total dissolved P was significantly correlated with DRP0.45 and on average DRP0.45 account for 76% of the TDP in runoff waters (Eq. 2).
The mass loss (g ha−1) of TDP was in the order 0.61 (Ni/MF) < 0.65 (Ni/PS) < 0.95 (Ni/CM) < 1.33 (Ni/DS) < 2.35 (DG/CS). The ratio DRP0.45 /TDP was ≈ 0.89 in all Ni treatments but in DG decreased to 0.75 highlighting the increase of organic P losses.
3.3 Changes in Soil Available P
Soil available P was evaluated by the Olsen method (Olsen et al., 1954). At the beginning of the experiment, Olsen P (mg kg−1) was 31 in Ni/MF, 37 in Ni/CM, 44 in Ni/PS, 49 in DG/CS and 87 in Ni/DS with significant differences (P < 0.001) between the treatments (Fig. 2). Nevertheless, all the plots were over fertilised with P (Olsen P > 23 mg kg−1, Horta et al., 2010). After the 2018–2019 crop cycle without P fertilisation, it was observed a significant decrease of Olsen P in all treatments (P < 0.001). Nevertheless, at the end of the experiment, Olsen P (mg kg−1) remained high with values of 23 mg kg−1 in Ni/CM, 25 in Ni/MF, 34 in Ni/PS, 43 in DG/CS and 67 in Ni/DS.
Olsen P level in soil before and after the experiment (Olsen P; mean ± SE); capital letters indicate statistical differences between the treatments for Olsen P before the experiment and small letters after the experiment. Different letters above the columns indicate significant differences between the treatments by the Duncan test at the P < 0.05 level
4 Discussion
4.1 Surface Runoff Waters and Sediments
The low rainfall during the crop cycle together with the role of ryegrass as a cover crop in preventing soil erosion should be the main reasons for the lack of sediments loss in all the treatments. Since the loss of sediments during the rainfall events was vestigial, the losses of P in particulate forms were negligible.
Overall, the amount of runoff waters during the crop cycle of all treatments was low. Nevertheless, DG/CS treatment showed the lower runoff amount compared with the other treatments which could be due to an increase of the soil infiltration rate. This trend was also observed in other works with organic amendment application to soil (Martinez et al., 2003; Spargo et al., 2006; Wester et al., 2011). In these works, it was also observed a decrease in the runoff water production which was highly related to the soil infiltration rate and also with the type of the organic amendment.
The pH values of the runoff waters were within the European surface water quality standards range of pH (Decree-Law nº236/98) and also within the range (pH of 6.5 to 9.0) tolerated for most aquatic biota (Novotny & Olen, 1994). The observed pH was similar to that reported by Rostagno and Sosebee (2001) and Cabrera et al. (2009) after biosolids (runoff water pH of 6.8–7.9) or dairy manure (runoff water pH of 6.6–6.8) application to the soil. These authors observed a decrease of the pH in the runoff water with the increasing rate of the dairy manure or biosolids application. In our experiment, there were no significant differences between the digestate and the Ni fertilisation. Losses of N, mainly the balance between N-NO3− and N-NH4+, could affect the runoff water pH. DG had 85% of the total N in organic forms and the mineralised organic N should have been uptake throughout the crop cycle with a similarly behave to other Ni treatments.
Concerning the loss of soluble salts, DG behaves like the inorganic N fertilisation treatments. Rostagno and Sosebee (2001) observed higher runoff EC values with the increased rate of biosolids application (from 0.1 to 0.6 dS m−1). In our experiment, the rate of N fertilisation was the same although through inorganic or organic sources which did not significantly affect the EC of the runoff waters between the treatments.
4.2 P Losses in Surface Runoff Waters
For all the treatments, the concentration of dissolved inorganic (DRP) and total dissolved P (TDP) in the runoff waters (Fig. 1) was higher than the threshold level to prevent the eutrophication of fresh water (0.05 mg Pt L−1, Golterman & Oude, 1991). Although the soil of all treatments was over fertilised with P, the sources of P seem to affect its losses to runoff waters. The treatments with past additions of different organic amendments or mineral fertilisation showed no significant differences between them. However, the current fertilisation with digestate increased the P losses to runoff waters compared with the Ni fertilisation irrespective to the past additions of organic amendments.
The DRP0.45 values in the runoff water of all treatments were within the range observed by other authors with application of biosolids (0.76–0.86 mg L−1, Martínez et al., 2003; Peyton et al., 2016), composts (0.3–0.5 mg L−1, Martínez et al., 2003; Spargo et al., 2006) and dairy cattle slurry (7.0 mg L−1, Peyton et al., 2016). Inorganic P was lost from soil to runoff waters mainly in dissolved forms and in very small particles of ø < 0.023 µm (DRP0.023 ≅ DRP0.45, Fig. 1a and Eq. 1). These forms of P pose the greatest environmental risk of eutrophication, because their orthophosphate composition is immediately algal-available (Pote & Daniel, 2000). Dodd et al. (2012) referred that 23 to 44 years would be needed to reduce the concentration of P in runoff waters to prevent the eutrophication from a soil over fertilised with P and with no further P fertiliser application. The soil of this experiment had a low P sorption capacity (Qmax = 95 mg P kg−1) and consequently P was accumulated mainly into labile forms with a high potential to be transferred to runoff waters even after 4 years of no P fertilisation. However, the increase of DRP in the runoff waters of DG/CS treatment compared with the other treatments should be due not only to the soil input of P from the digestate (digestate had a content of 11.7 g P kg−1 DM) but also to the decrease of soil P sorption. The latter would be achieved through the competition of the organic acids originated during the degradation of the OM of the digestate for the same soil P sorption sites as observed in other works (Horta & Carneiro, 2020; Nest et al., 2016; Nziguheba et al., 1998). In addition, most of Pi forms in the digestate (60% of the total Pi fractions) were in easily available forms that is P with high mobility to the transfer from soil to runoff waters. DG showed also an increase of organic P losses for runoff waters observed by the decrease of the ratio DRP0.45/TDP (≈ 0.89 in all Ni treatments to 0.75 in DG). Once more DG showed the higher mass loss (2.35 g ha−1) of TDP compared with the other treatments. Nevertheless, the mass loss of TDP observed in all treatments was lower than those referred for the European Union (European Commission, 2013) from 0.1 to higher than 1.0 kg P ha−1 year−1. The low rainfall as explained above could justify our low values of P mass loss since the concentration of P in runoff waters was very high.
4.3 Changes in Soil Available P
At the beginning of the experiment, all of the plots are over fertilised with P and as referred above all provided high transfer of dissolved P to runoff waters. Despite DG/CS had the lowest past P addition (50 compared with 100 kg ha−1 in the other treatments, Table 1), it was also over fertilised with P although this treatment had not the highest initial soil Olsen P content. Since the N fertilisation was done only through the digestate which had an N/P ratio of 2.3:1 that was quite low regarding crop P requirement, the fertilisation done added a surplus of P. Consequently, DG/CS showed not only the higher P losses to runoff waters but also the lower decrease of Olsen P during the crop cycle. This behaviour seems to confirm that the P content of the digestate and also the effect of digestate in mobilising soil sorbed P to available forms (Horta & Carneiro, 2020; Nest et al., 2016; Nziguheba et al., 1998) can be an advantage in agronomic nutrient’s management but poses a risk in the environmental management of soil P legacy.
5 Conclusions
The main pathway of P loss from this over fertilised dystric Regosol was by surface runoff waters. The P losses occurred in dissolved forms and in particles of ø < 0.023 µm (DRP0.023) which poses the greatest environmental risk of eutrophication since this orthophosphate forms are immediately algal available. In addition, the concentration of total dissolved P (TDP) in the runoff waters of all treatments was always higher than 0.05 mg L−1 considered the threshold level to prevent eutrophication of fresh waters. On average, TDP ranged between 0.5 (Ni/PS) and 2.6 mg L−1 (DG/CS). Irrespective to the past P additions to soil (from inorganic fertilisers or from organic amendments), the losses of P were mainly in inorganic P forms. The digestate application increased the losses of P to runoff waters both in inorganic and organic forms. During the experiment from December 2018 to May 2019, the rainfall was lower (212 mm) than the climatological normal for the region (433 mm) and runoff water was also low in all treatments and ranged between 412 L ha−1 in DG/CS and 1155 L ha−1 in Ni/DS. Consequently, the mass losses of TDP were low and ranged between 0.61 (Ni/MF) and 2.35 g ha−1 (DG/CS). Halting P fertilisation originated a decrease in soil Olsen P in all treatments: 5.5 (DG/CS) < 6.1 (Ni/MF) < 10.0 (Ni/PS) < 13.5 Ni/CM < 20.0 mg kg−1 (Ni/ DS). Sediments loss was also negligible, which did not allow to evaluate the loss of P by soil erosion. At the end of the experiment, Olsen P in Ni/MF was 25 mg kg−1 and in Ni/CM 23 mg kg−1 closer to 20 mg kg−1 considered the threshold level for agronomic purposes. Nevertheless, the concentration of P in runoff waters remained high in all treatments and the fertilisation with digestate increased not only the risk of P losses to fresh waters but also prevented the decrease of the available P in the over fertilised soil.
Data Availability
The data of the present work is available for download from the “Repositório Científico do Instituto Politécnico de Castelo Branco” (Scientific Repository of the Polytechnic Institute of Castelo Branco) at the following link: http://hdl.handle.net/10400.11/7549.
References
AFNOR: NF U44–051. (2006). Amendments organiques—Dénominations, specifications et marquage.
AFNOR: FD CR 13456. (2001). Amendments du sol et supports de culture—Etiquetaje, spécifications et listes de produits.
American Public Health Association (APHA). (2012). Standard methods for the examination of water and wastewater. 22nd Ed. American Water Works Association (AWWA), Water Environment Federation (WEF) and American Public Health Association, Washington, DC, USA. 1360 p.
Ayers, R.S. & Westcott, D. W. (1994). Water quality for agriculture. FAO Irrigation and Drainage Paper. No. 29 rev1. FAO, Rome. 180 p.
Brandt, R.C., Elliot, H. A., & O’Connor, G. A. (2004). Water-extractable phosphorus in biosolids: Implications for land-based recycling. Water Environment Research, 76, 121–129. https://www.jstor.org/stable/25045757. Accessed 27 Feb 2021.
Borggaard, O. K., Raben-Lange, B., Gimsing, A. L., & Strobel, B. W. (2004). Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma, 127, 270–279. https://doi.org/10.1016/j.geoderma.2004.12.011
Cabrera, V. E., Stavast, L. J., Baker, T. T., Wood, M. K., Cram, D. S., Flynn, R. P., & Ulery, A. L. (2009). Soil and runoff response to dairy manure application on New Mexico rangeland. Agriculture, Ecosystems and Environment, 131, 255–262. https://doi.org/10.1016/j.agee.2009.01.022
Decree-Law 236/1998. Decreto Lei 236/1998, Diário da República, 1ª série – nº 176 – 1 de Agosto de 1998.
Decree-Law 306/2007. Decreto Lei 306/2007, Diário da República, 1ª série – nº 164 – 27 de Agosto de 2007.
Decree-Law 103/2015. Decreto Lei 103/2015, Diário da República, 1ª série – nº 114 – 15 junho de 2015.
Deshpande, V. V., & Telang, M. S. (1950). Pipet method of sedimentation analysis. Rapid determination of distribution of particle size. Analytical Chemistry, 22, 840–841. https://doi.org/10.1021/ac60042a033
Dodd, R. J., McDowel, R. W., & Condron, L. M. (2012). Predicting the changes in environmentally and agronomically significant phosphorus forms following the cessation of phosphorus fertilizer applications to grasslands. Soil, Use and Management, 28, 135–147. https://doi.org/10.1111/j.1475-2743.2012.00390.x
Elliot, H. A., Brandt, R. C., & O’Connor, G. A. (2005). Runoff phosphorus losses from surface-applied biosolids. Journal of Environmental Quality, 34, 1632–1639. https://doi.org/10.2134/jeq2004.0467
Eghball, B. (2002). Soil properties as influenced by phosphorus- and nitrogen-based manure and compost applications. Agronomy Journal, 94, 128–135. https://doi.org/10.2134/agronj2002.1280
Egnér, H., Riehm, H., & Domingo, W. R. (1960). Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor und Kaliumbestimmung. Kung Lantbrukshögskolans Annaler, 26, 199–215.
EUR-Lex—52015DC0614—EN—EUR-Lex. (2015). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Closing the loop - an EU Action Plan for the Circular Economy. European Commission, Brussels. 21pp. online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52015DC0614. Accessed 25 Feb 2021.
European Commission. (2013). Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Consultative Communication on the Sustainable Use of Phosphorus. online: https://ec.europa.eu/environment/consultations/pdf/phosphorus/EN.pdf [accessed 25/02/2021].
European Commission. (2018). A sustainable bioeconomy for Europe: Strengthening the connection between economy, society and the environment: Updated bioeconomy strategy. online: https://ec.europa.eu/research/bioeconomy/pdf/ec_bioeconomy_strategy_2018.pdf [accessed 55/02/2021].
Fox, R. L., & Kamprath, E. J. (1970). Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Science Society of America, Proceedings, 34, 903–906. https://doi.org/10.2136/sssaj1970.03615995003400060025x
Frossard, E., Brossard, M., Hedley, M. J., & Metherell, A. (1995). Reactions controlling the cycling of P in soils. In H. Tiessen (Ed.), Phosphorus in the global environment (pp. 107–137). John Wiley & Sons.
Gagnon, B., Demers, I., Ziadi, N., Chantigny, M. H., Parent, L.-E., Forge, T. A., Larney, F. J., & Buckley, K. E. (2012). Forms of phosphorus in composts and in compost-amended soils following incubation. Canadian Journal of Soil Science, 92, 11–721. https://doi.org/10.4141/cjss2012-032
García-Albacete, M., Tarquis, A. M., & Cartagena, M. C. (2014). Risk of leaching in soils amended by compost and digestate from municipal solid waste. The Scientific World Journal, 2014, 1–9. https://doi.org/10.1155/2014/565174
Gerke, J., & Hermann, R. (1992). Adsorption of orthophosphate to humic-Fe-complexes and to amorphous Fe-oxide. Journal of Plant Nutrition and Soil Science, 155, 233–236. https://doi.org/10.1002/jpln.19921550313
Golterman, H. L., & Oude, N.T. (1991). Eutrophication of lakes, rivers and coastal seas. In: The handbook of environmental chemistry, Volume 5 Part A, pp 80–124. Ed. O. Hutzinger. Berlin, Springer-Verlag.
Horta, M.C., & Torrent, J., (2007a). Phosphorus desorption kinetics in relation to phosphorus forms and sorption properties of Portuguese acid soils. Soil Science, 172, 631−638. http://0038-075X/07/17208-631-638
Horta, C., & Torrent, J. (2007). The Olsen P method as an agronomic and environmental test for predicting phosphate release from acid soils. Nutrient Cycling in Agroecosystems, 77, 283–292. https://doi.org/10.1007/s10705-006-9066-2
Horta, C., Roboredo, M., Coutinho, J., & Torrent, J. (2010). Relationship between Olsen P and ammonium lactate–extractable P in Portuguese acid soils. Communications in Soil Science and Plant Analysis, 41, 2358–2370. https://doi.org/10.1080/00103624.2010.508296
Horta, C., Roboredo, M., Carneiro, J. P., Duarte, A. C., Torrent, J., & Sharpley, A. (2018). Organic amendments as a source of phosphorus: Agronomic and environmental impact of different animal manures applied to an acid soil. Archives of Agronomy and Soil Science, 63, 257–271. https://doi.org/10.1080/03650340.2017.1346372
Horta, C., & Carneiro, J. P. (2020). Assessment of fertilising properties of a solid digestate in comparison with undigested cattle slurry applied to an acidic soil. Open Journal of Soil Science, 10, 307–325. https://doi.org/10.4236/ojss.2020.108017
Humphreys, M. O. (2005). Genetic improvement of forage crops – Past, present and future. The Journal of Agricultural Science, 143, 441–448. https://doi.org/10.1017/S0021859605005599
[INIAP] – Laboratório Químico Agrícola Rebelo da Silva. (2006). Manual de Fertilização das Culturas. Lisboa, INIAP. 282 p.
IUSS Working Group WRB. (2015). World Reference Base for Soil Resources 2014, update 2015 international soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106, FAO, Rome. 192 p.
JRC Scientific and Policy Reports. (2014). End-of-waste criteria for biodegradable waste subjected to biological treatment (compost & digestate): Technical proposals. online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC87124/eow%20biodegradable%20waste%20final%20report.pdf [accessed 25/02/2021].
Kleinman, P. J. A., & Sharpley, A. N. (2003). Effect of broadcast manure on runoff phosphorus concentrations over successive rainfall events. Journal of Environmental Quality, 32, 1072–1081. https://doi.org/10.2134/jeq2003.1072
Martínez, F., Casermeiro, M. A., Morales, D., Cuevas, G., & Walter, I. (2003). Effects on runoff water quantity and quality of urban organic wastes applied in a degraded semi-arid ecosystem. Science of the Total Environment, 305, 13–21. https://doi.org/10.1016/S0048-9697(02)00472-2
Matar, A., Torrent, J., & Ryan, J. (1992). Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. Advances in Soil Science, 18, 81–146. https://doi.org/10.1007/978-1-4612-2844-8_3
McDowell, R., Dodd, R., Pletnyakov, P., & Noble, A. (2020). The ability to reduce soil legacy phosphorus at a country scale. Frontiers in Environmental Science, 8, 6. https://doi.org/10.3389/fenvs.2020.00006
Möller, K., Schulz, R., & Müller, T. (2010). Substrate inputs, nutrient flows and nitrogen loss of two centralized biogas plants in southern Germany. Nutrient Cycling in Agroecosystems, 87, 307–325. https://doi.org/10.1007/s10705-009-9340-1
Murphy, J., & Riley, J. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nest, T. V., Ruysschaert, G., Vandecasteele, B., S. Houot, S., Baken, S., Smolders, E., Cougnon, M., Reheul D., & Merck, R. (2016). The long term use of farmyard manure and compost: Effects on P availability, orthophosphate sorption strength and P leaching. Agriculture, Ecosystems and Environment, 201, 23−33https://doi.org/10.1016/j.agee.2015.09.009
Novotny, V., & Olen, H. (1994). Water quality – Prevention, identification, and management of diffuse pollution (p. 1054). Van Nostrand Reinhold Publishers.
Nziguheba, G., Palm, C. A., Buresh, R. J., & Smithson, P. C. (1998). Soil phosphorus fractions and adsorption as affected by organic and inorganic sources. Plant and Soil, 198, 159–168. https://doi.org/10.1023/A:1004389704235
Olsen, S., Cole, C., Watanabe, F., & Dean, L. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate, Circular 939, U.S. Department of Agriculture, Washington DC.
Peyton, D. P., Healy, M. G., Fleming, G. T. A., Grant, J., Wall, D., Morrison, L., Cormican, M., & Fenton, O. (2016). Nutrient, metal and microbial loss in surface runoff following treated sludge and dairy cattle slurry application to an Irish grassland soil. Science of the Total Environment, 541, 218–229. https://doi.org/10.1016/j.scitotenv.2015.09.053
Pote, D. H. & Daniel, T. C. (2000). Analyzing for dissolved reactive phosphorus in water sample. p. 91–93. In: G. M. Pierzynski (Ed.). Methods of phosphorus analysis for soils, sediments, residuals, and waters. Southern Cooperative Series Bulletin No. 396. Southern Association of Agricultural Experiment station Directors. Raleigh, NC.
Rostagno, C. M., & Sosebee, R. E. (2001). Biosolids application in the Chiuahuan Desert: Effects on runoff water quality. Journal of Environmental Quality, 30, 160–170. https://doi.org/10.2134/jeq2001.301160x
Siebert, S. (2008). Quality requirements and quality assurance of digestion residuals in Germany. ECN/ORBIT Workshop the Future for Anaerobic Digestion of Organic Waste in Europe, Nuremberg. online: http://www.kompost.de/uploads/media/Quality_Requirements_of_digestion_residu als_in_Germany_text_01.pdf [accessed 25/02/2021].
Soltangheisi, A., Teles, A. P. B., Sartor, L. R., & Pavinato, O. S. (2020). Cover cropping may alter legacy phosphorus dynamics under long-term fertilizer addition. Frontiers in Environmental Science, 8, 13. https://doi.org/10.3389/fenvs.2020.00013
Spargo, J. T., Evanylo, G. K., & Alley, M. M. (2006). Repeated compost application effects on phosphorus runoff in the Virginia Piedmont. Journal of Environmental Quality, 35, 2342–2351. https://doi.org/10.2134/jeq2006.0105
Tambone, F., Genevini, P., D’Imporzano, G., & Adani, F. (2009). Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresource Technology, 100, 3140–3142. https://doi.org/10.1016/j.biortech.2009.02.012
Tani, M., Sakamoto, N., Kishimoto, T., Umetsu, K., & K. . (2006). Utilization of anaerobically digestate slurry combined with other waste following application to agricultural land. International Congress Series, 1293, 331–334. https://doi.org/10.1016/j.ics.2006.03.013
Teglia, C., Tremier, A., & Martel, J.-L. (2011). Characterization of solid digestates: Part I, review of existing indicators to assess solid digestates agricultural use. Waste and Biomass Valorization, 2, 43–58. https://doi.org/10.1007/s12649-010-9051-5
Torrent, J. (1997). Interactions between phosphate and iron oxide. In K. Auerswald (Ed.), Soils and environment, Advances in GeoEcology 30 (pp. 321–344). Germany, CATENA Verlag.
Traoré, O., Sinaj, S., Frossard, E., & van de Kerkhove, J. M. (1999). Effect of composting time on phosphate exchangeability. Nutrient Cycling in Agroecossystems, 55, 123–131. https://doi.org/10.1023/A:1009828927161
Walkley, A., & Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37, 29–38. https://doi.org/10.1097/00010694-193401000-00003
Wester, D. B., Sosebee, R. E., Zartman, R. E., Fish, E. B., Villalobos, J. C., Mata-Gonzalez, R., Jurado, P., & Moffet, C. A. (2011). Biosolids effects in Chihuahuan desert rangelands: A ten-year study. Applied and Environmental Soil Science, 2011, 1–13. https://doi.org/10.1155/2011/717863
Voća, N., T. Krička, T., Ćosić, T., Rupić, V., Jukić, Ž., & Kalambura, S. (2005). Digestate residue as a fertilizer after mesophilic process of anaerobic digestion. Plant, Soil and Environment, 51, 262−266https://doi.org/10.17221/3584-PSE
Acknowledgements
This work was carried out under the project INTERREG 0340_SYMBIOSIS_3_E.
Funding
This research was funded by the QRural – Quality of Life in the Rural World – Research Unit, Polytechnic Institute of Castelo Branco, Castelo Branco, Portugal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Horta, C., Carneiro, J. Phosphorus Losses to Surface Runoff Waters After Application of Digestate to a Soil Over Fertilised with Phosphorus. Water Air Soil Pollut 232, 439 (2021). https://doi.org/10.1007/s11270-021-05382-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05382-y