Abstract
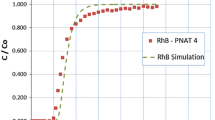
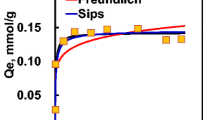
Aqueous phase adsorption of three textile dyes onto a granular activated carbon produced from acid activation of almond shells is presented. Primarily, the sorption of three basic dyes, methylene blue, rhodamine b, and malachite green oxalate were studied. Four models, the Freundlich, the Langmuir, the Redlich-Peterson, and the Toth isotherms were compared for their quality of fit to the single-solute sorption data. Next, sorption of the three likely binary systems was examined. Four bi-solute models, the extended Langmuir with and without an interaction term, the extended Redlich-Peterson with an interaction term, and the empirical extended Freundlich model were used to predict sorption in the binary systems. Nonlinearly determined constants of the corresponding single-solute isotherms were used in the binary models to compare with experimental binary sorption data. For the single-solute system, the three-parameter models of the Redlich-Peterson and the Toth isotherms outperformed the Langmuir and Freundlich models. The empirical extended Freundlich model produced the closest comparison to the binary data in each system. In general, the nonlinear method provided a simple and computationally effective technique of producing optimal fitting parameters for the bi-solute sorption models.




Similar content being viewed by others
References
Al-Duri, B., Magdy, Y., Saad, R., & McKay, G. (1991). Modeling of multi-component equilibrium for the adsorption of basic dyes onto bagasse pith. Transactions of the Institution of Chemical Engineers, 69, 246–254.
Allen, S. J., McKay, G., & Porter, J. F. (2004). Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. Journal of Colloid and Interface Science, 280, 322–333.
Chevalier, L. R., Yesuf, J. N., & Knowles, L. S. (2007). Evaluation of textile dye sorption on GAC produced from almond shells based on optimized isotherm models. Environmental Engineering Science, 24(4), 563–579.
Dastgheib, S. A., & Rockstraw, D. A. (2002). A systematic study and proposed model of the adsorption of binary metal ion solutes in aqueous solution onto activated carbon produced from pecan shells. Carbon, 40, 1853–1861.
Fylstra, D., Lasdon, L., Watson, J., & Waren, A. (). ()). Design and use of the Microsoft Excel Solver. Interfaces, 28(5), 29–55.
Fritz, W., & Schluender, E. U. (1974). Simultaneous adsorption equilibria of organic solutes in dilute aqueous solutions on activated carbon. Chemical Engineering Science, 29, 1279–1282.
Gupta, V. K., Mittal, A., & Gajbe, V. (2005). Adsorption and desorption studies of a water soluble dye, quinoline yellow, using waste materials. Journal of Colloid and Interface Science, 284, 89–98.
Lasdon, L. S., Waren, A. D., Jain, A., & Ratner, M. (1978). Design and testing of a generalized reduced gradient code for nonlinear programming. ACM Transactions on Mathematical Software, 4(1), 34–49.
McKay, G., & Al-Duri, B. (1989). Prediction of multicomponent adsorption equilibrium data using empirical correlations. The Chemical Engineering Journal, 41, 9–23.
Namane, A., Mekarzia, A., Benrachedi, K., Belhaneche-Bensemra, N., & Hellal, A. (2005). Determination of the adsorption capacity of activated carbon made from coffee grounds by chemical activation with ZnCl2 and H2PO4. Journal of Hazardous Materials, 119, 189–194.
Porter, J. F., McKay, G., & Choy, K. H. (1999). The prediction of sorption from a binary mixture of acidic dyes using single and mixed-isotherm variants of the ideal adsorbed solute theory. Chemical Engineering Science, 54, 5863–5885.
Radke, C. J., & Prausnitz, J. M. (1972). Adsorption of organic solutes from dilute aqueous solution on activated carbon. Industrial and Engineering Chemistry Fundamentals, 11(4), 445–451.
Toles, C. A., MArshall, W. E., & Johns, M. M. (1997). Granular activated carbons from nutshells for the uptake of metals and organic compounds. Carbon, 35(9), 1407–1414.
Weber, W. J. Jr, & DiGiano, F. A. (eds.) (1996). Process dynamics in environmental systems. New York: John Wiley and Sons.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yesuf, J.N., DeVantier, B.A. & Chevalier, L.R. Bisolute Equilibrium Studies for the Sorption of Basic Dyes on a GAC from Almond Shells: A Nonlinear Approach. Water Air Soil Pollut: Focus 8, 387–393 (2008). https://doi.org/10.1007/s11267-007-9156-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11267-007-9156-4