Abstract
We report a novel reovirus (MRV-HLJ/2007) isolated from swine in Heilongjiang Province, China. Genome sequence analysis indicated a close genetic relationship between MRV-HLJ/2007 and strain SC-A, which was isolated from swine in 2006 in Sichuan, China. Although phylogenetic analysis indicated that MRV-HLJ/2007 may have originated from the SC-A strain, the M2 and S3 genes differ between these strains. Phylogenetic analysis also showed that, except for differences in the S1 gene, MRV-HLJ/2007 and SC-A are closely related to a reovirus that infects humans. These findings suggest that MRV-HLJ/2007 might be a novel reovirus strain circulating in China.
Similar content being viewed by others
Introduction
Reoviruses (respiratory enteric orphan viruses) represent a large and diverse group of nonenveloped viruses with segmented dsRNA genomes and are taxonomically classified into 15 genera in the family Reoviridae [1]. Members of the genus Orthoreovirus contain 10 genome segments and have been isolated from a broad range of mammalian, avian, and reptilian hosts. The mammalian reoviruses (MRVs) including the Orthoreovirus were first described as ‘‘respiratory and enteric orphans’’ in the United States and Mexico in the 1950s [2, 3].Three MRV serotypes have been recognized based on the capacity of anti-reovirus sera to neutralize viral infectivity and inhibit hemagglutination (HA). More recent sequence-based analyses support the placement of most MRVs into the three classically recognized serotypes, in which type 1 Lang (T1L), type 2 Jones (T2J), and type 3 Dearing (T3D) are representative of the three serotypes infecting humans [4–6].
MRV infections in humans are generally benign with very rare cases of mild upper respiratory tract illness or enteritis in infants and children [7]. Many diseases in nonhuman animals have also been attributed to MRV infection, from neurological symptoms in baboons and snakes to pneumonia and death in chickens. All three serotypes of MRV have been isolated from swine, and experimental infection with serotype I has been reported to cause swine enteritis and pneumonia [8–10]. An MRV has been isolated from swine in Sichuan province of southwestern China, and the whole genome has been deposited in Genbank [11].
Here, we report the identification and characterization of a novel MRV-3 strain (MRV-HLJ/2007) isolated from swine with fever and acute respiratory illness resembling the Porcine Respiratory Disease Complex (PRDC). We also provide evidence suggesting that most genes of the MRV-HLJ/2007 strain originated from the Sichuan strain (SC-A) of MRV, but that MRV-HLJ/2007 is closely related to the other MRV strains based on analysis of the M2 and S3 gene. The results indicate that MRV-HLJ/2007 might be a new MRV-3 strain in China.
Materials and methods
Samples
Samples were taken from a disease outbreak in a swine farm in Heilongjiang Province, northeastern China, in May 2007. One hundred and twenty piglets (50 days old) had been introduced into a swine-fattening farm with more than 1,000 pigs. One week after the introduction of the new piglets, approximately 100 piglets presented with clinical signs resembling porcine respiratory disease complex (PRDC). Specimens including spleen and tonsils were collected from three dead piglets (between 50 and 70 days old) that had exhibited diarrhea and respiratory distress. Fecal samples were collected from 10 other symptomatic piglets. Contamination was avoided by collection of the samples directly from the anus and using disposable materials. In addition, serum samples from 45 symptomatic piglets were collected, frozen, and sent for analysis to the State Key Laboratory of Veterinary Biotechnology at the Harbin Veterinary Research Institute. Finally, specimens of livers, spleens, and lungs from 10 adult swine that died from the disease were also sent to the same laboratory.
Virus isolation
Swine testicular (ST) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS; PAA Lab GmbH, Pasching, Austria), 100 U/ml of penicillin G, 100 μg/ml of streptomycin, and 2.5 μg/ml of amphotericin B. The ST cells were grown at 37°C in a humidified 5% CO2 atmosphere.
Before the pooled samples from the three dead piglets were ground (the pooled spleen samples and pooled tonsil samples were ground separately), DMEM culture containing ST cells was added (1/20, w/v). The ground material was frozen and thawed three times. After the mixture was centrifuged at 1,500 rpm for 10 min, the supernatant was collected and antibiotics were added. The supernatant was diluted at 1:9 in medium. Then, the supernatant was used to inoculate the ST cells, which were cultured at 37°C in a humidified 5% CO2 atmosphere.
The culture underwent at least three cell culture passages in the ST cell line before being reported as negative. Maintenance medium was replenished at day 7, and cultures were terminated 14 days after inoculation. All cultures were observed daily for cytopathic effect (CPE). The cell cultures that had a clear CPE and that tested positive for MRV by RT-PCR were subjected to morphological analysis, partial genome sequencing, and serological analysis. The isolated strain was named MRV-HLJ/2007 and identified by PCR.
Bacteria identification
The presence of pathogenic bacteria in fecal samples was also determined by standard aerobic and anaerobic bacterial culture testing procedures using pre-reduced Columbia agar and Mac-Conkey’s agar. After a 96-h incubation, different colony types were subcultured and then subjected to phenotypic and biochemical identification. The presence of Mycoplasma, Streptococcus, and Listeria was tested by PCR [12].
Electron microscopy
A portion (100 ml) of the MRV-HLJ/2007 virus culture was clarified by centrifugation at 2,000×g for 10 min followed by 200,000×g for 3 h. The resulting pellet was resuspended in a small amount of phosphate-buffered saline (PBS) and nebulized onto coated EM grids. The grids were stained with 1% phosphotungstic acid (pH 7.0) and observed with a transmission electron microscope (JEOL-JSM-1200) for morphological identification of the virus.
Immunoperoxidase monolayer assay (IPMA)
The 45 serum samples from diseased piglets were tested for antibodies to respiratory syndrome virus (PRRSV), swine influenza virus (SIV), and porcine circovirus (PCV) by IPMA. These viruses were selected because each is known to cause symptoms in swine resembling those observed in this outbreak [13]. When the virus was identified as a serotype 3 reovirus by RT-PCR, the sera were further tested for MRV-3 antibody.
Plates with 96 wells containing MRV-HLJ/2007 and mock-infected cells were fixed in 33.3% acetone for 20 min at room temperature and dried (stored at −70°C). The cells were blocked with PBS containing 5% (w/v) BSA for 12 h at 4°C. A 50-μl volume of each serum sample was added to the MRV-infected and noninfected cells, which were then incubated at 37°C for 1 h. After the unbound antibodies were washed three times with PBS, an optimum dilution (1:3,000) of horseradish peroxidase-conjugated Protein A was added, and the samples were incubated for 30 min at 37°C. After washing, color development was carried out with 3-amino-9-ethylcarbazole and hydrogen peroxide in 0.05 M acetate buffer (pH 5.0) for 30 min at room temperature. The reaction was terminated by removal of the substrate. After they were washed once with distilled water, the cells were dried again and then examined with an inverted light microscope. Serum samples that gave a positive signal were at a serum dilution of 1:40 or higher were scored as positive.
Reverse transcription-polymerase chain reaction assay (RT-PCR assay)
Total RNA was extracted from the isolated virus with TRIzol reagent according to the manufacturer’s instructions (Invitrogen), and RT-PCR was performed according to the manufacturer’s instructions (SuperScript III, Invitrogen) and as described in previous reports concerning PRRSV, PRV, and SIV [14–16]. For the detection of MRV, a nested-PCR assay was used [17–19].
Indirect immunofluorescence assay (IFA)
For the detection of IgG specific to MRV, serum samples were tested by an “in-house” indirect immunofluorescence assay (IFA) as described by Wulff et al. [20] with some modifications. ST cells were inoculated with the MRV-3 strain and were cultured for 72 h. Infected cells were harvested and mixed with 0.5-fold of noninfected cells, washed in PBS, spotted on slides, and fixed with ice-cold acetone. Fixed slides were stored at −70°C until used. All serum samples were screened at a dilution of 1:40 in PBS. A 50-μl volume of each serum sample was spotted per well and incubated for 30 min at 37°C. After slides were washed for 10 min in PBS, 20 μl of fluoroisothiocyanate-conjugated anti-porcine IgG diluted 1:40 in buffer containing Evans blue was added to each well and incubated for 30 min. The contents of each well was then embedded in glycerin and examined by immunofluorescence microscopy. Positive samples were further tested in twofold serial dilutions to determine the endpoint titer. A titer of 1:20 was considered positive. The specificity of the IFA has been previously demonstrated (unpublished data).
Sequencing and phylogenetic analysis
cDNAs were amplified with Platinum Taq High Fidelity polymerase (Invitrogen, SanDiego, CA). PCR were subjected to 35 cycles of denaturation for 45 s at 94°C, annealing for 1 min at 55°C, and extension for 2 min at 72°C, followed by a final extension cycle at 72°C for 10 min. The amplified products were purified by the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and directly sequenced by the BigDye Terminator Cycle chemistry on an ABI 3100-Avanti Genetic Analyzer using the primers listed in Supplementary Table 1.
The whole genome of MRV-HLJ/2007 was amplified, and the PCR products were sequenced by the ABI PRISM 3730 DNA sequencer (Applied Biosystem). The sequence of MRV-HLJ/2007 was compared with other published MRV sequences. Sequence alignments were generated by the ClustalX software. Phylogenetic analyses were performed with the neighbor-joining (NJ) methods in MEGA software package version 4.0.2. Branching orders of the phylograms were verified statistically by resampling the data 1,000 times in a bootstrap analysis with the branch-and-bound algorithm as applied in MEGA.
Results
Virus isolation and morphology
After five passages of the isolated virus on ST cells, a distinct cytopathic effect was observed, and after three additional passages, the virus titer was greater than 107.5 TCID50/ml. After five passages, cell cultures showed positive results when tested by IPMA. IPMA was also performed with sera from 45 diseased piglets; 40 samples were positive for MRV antibody, three samples were positive for PCV2 antibody, and no sample was positive for PRRSV or SIV antibody. Electron microscopy of a negatively stained sample of the isolated virus revealed a small virus with a diameter of 60–80 nm (Fig. 1). The viruses were naked and had a double capsid with a conspicuous spike and turret in the core.
Bacteria identification
Bacteria were cultured from the fecal samples and identified based on phenotypic and biochemical characteristics. Bacteria that could not be identified based on these characteristics were subjected to PCR. All samples were negative for Mycoplasma, Streptococcus, and Listeria.
IFA and RT-PCR assay
Of the 45 sera samples from diseased piglets, 43 reacted positively in the MRV-3-specific IFA (Fig. 2). For RT-PCR analyses, all fecal, organ, and serum samples were first examined for the presence of PRSV, SIV, PCV-2, and MRV. All assays were negative except for three samples that were PCV2 positive. All fecal and spleen samples were then subjected to RT-PCR; MRV was detected in 12 out of 13 spleen samples (three out of three samples were positive from the dead piglets, and nine out of 10 samples were positive from the dead adults), and MRV was detected in eight of ten fecal samples (Supplementary Table 2). Among the 45 serum samples from diseased piglets, 37 were positive in the MRV-specific RT-PCR assay. The PCR product was sequenced and found to belong to the MRV serotype 3.
Sequencing and phylogenetic analysis
The MRV-HLJ/2007 L1–L3, M1–M3, and S1–S4 genes were amplified and sequenced (GeneBank Accession Numbers: HQ642769-HQ642778). The reovirus S1 gene encodes the virus attachment protein σ1 [21]. The viral σ1 protein, which is unique to each prototype of mammalian reovirus, determines the serotype and is also the major genetic determinant of neurovirulence in infected mice.
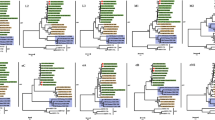
The sequence of the S1 gene of MRV-HLJ/2007 was compared with the sequences available in Genbank. Phylogenetic trees produced very similar topologies with the maximum-likelihood and NJ methods. An NJ tree of 39 sequences is presented in Fig. 3. The sequences were classified into three genotypes, which represent the three serotypes of the reoviruses. The phylogenetic analysis based on the S1 gene indicated that the diversity of the reoviruses is dependent on their serotype. The phylogenetic tree also showed that there is a higher divergence in serotype 3 reovirues from different hosts than in serotype 1 or 2.
The sequences in serotype 3 reovirus fall into four main subgroups based on phylogenetic analysis of the S1 gene. The sequences in the subgroup including MRV-HLJ/2007 were all from strains isolated in China. Both MRV-HLJ/2007 and SC-A were isolated from swine. The MPC/04 strains were isolated from masked palm civets. According to a phylogenetic tree based on the S1 gene, MRV-HLJ/2007 is closely related to the MPC/04 strain and SC-A strain. The S1 gene of MRV-HLJ/2007 showed 92% identity with the S1 gene of SC-A and 95% identity with the S1 gene of MPC/04. The predicted product of the S1 gene of MRV-HLJ/2007 (σ1 protein) contained 447 amino acids and showed 94% identity with MPC/04, 97% identity with SC-A, 51% identity with prototype T2J reovirus, and 49% identity with T1L reovirus. The phylogenetic trees showed that the MRV-HLJ/2007 is much more closely related to MPC/04 than to SC-A.
We have also compared the rest of the genes of MRV-HLJ/2007 with the corresponding genes available in the GenBank (Supplementary Figures S1–S7). The S3 and M2 gene of MRV-HLJ/2007 are divergent from those of the SC-A strain, while the other genes are more closely related to those of the SC-A strain (Figs. 4, 5). The S3 gene of MRV-HLJ/2007 showed 82% identity with that of SC-A and 77% with that of MPC/04. The predicted product of the S3 gene of MRV-HLJ/2007 (sigma-NS protein) contained 253 amino acids and showed highest identity (97 and 96%) to the product of SC-A and MPC/04, respectively. The M2 gene of MRV-HLJ/2007 showed 88% identity with that of SC-A and 92% with that of MPC/04. The predicted product of the M2 gene of MRV-HLJ/2007 (mu-1 protein) contained 476 amino acids and showed highest identity (98% and 98%) to the M2 products of SC-A and MPC/04, respectively. These results indicate that MRV-HLJ/2007 may be a novel strain circulating in China.
The phylogenetic results showed that the S2, S4, L1, L2, L3, M1, M2, and M3 genes of MRV-HLJ/2007 and SC-A are closely related to those genes of the MRV2Tou05 strain, which was isolated from a human (See Supplementary Figures S1–S9). Except for S4, the genes of SC-A are more closely related than the genes of MRV-HLJ/2007 to those of MRV2Tou05. The phylogenetic analysis of the S3 gene indicates a close evolutionary relationship between MRV-HLJ/2007 and MRV2Tou05 and that SC-A separated from the subgroup that included MRV-HLJ/2007. Based on the analysis of the S1 gene, MRV2Tou05 is much more closely related to the 302I and 302II strains than to the other strains tested. The 302I and 302II strains were isolated from fecal specimens of two children in Beijing in 1982 [22]. We infer that MRV2Tou05 might therefore be a human–swine reassortant virus.
Discussion
The reoviruses analyzed in this study were isolated from swine. The virus that was isolated from sick and dead swine in this study appears to be a genetically distinct reovirus. To date, there are few reports on the epidemiology of reoviruses in swine, although all the three MRV serotypes have been isolated from humans, dogs, and many other species [1, 2]. Electron microscopy revealed a negatively stained virus with a diameter of 60–80 nm. The viruses were naked and possessed a double capsid with a conspicuous spike and turret in the core. IMPA revealed that 90% of the serum specimens contained reovirus (MRV-3) antibody, while IFA revealed that 95% of serum samples contained MRV-3 antibody. The isolated virus was further identified as a serotype 3 reovirus by PCR and sequencing.
In China, reovirus 3 serotype (MRV-3) has been previously isolated from swine, but the isolated MRV-3 strain was not molecularly characterized. In the current study, we sequenced the whole genome of the newly isolated strain MRV-HLJ/2007 and analyzed its phylogenetic relationship with published strains. The S1 genome segment is unique to each MRV strain. The S1 segment encodes protein σ1 and nonstructural protein σ1s, while the other nine segments generally encode just one protein. Protein σ1 is responsible for cell attachment, specifying tissue tropism, and hemagglutination. The S1 sequence shows the greatest diversity of all genome segments, with only 26–56% identity between viruses belonging to different serotypes. In contrast, S1 sequence identity between viruses of the same serotype ranges from 86 to 99%, which is similar to the sequence identity of the other genome segments between viruses belonging to different serotypes [23]. The predicted product of the MRV-HLJ/2007 S1 gene (σ1 protein) contained 447 amino acids. The S1 sequence of MRV-HLJ/2007 had the highest identity (93 and 98%) with MPC/04 and SC-A, 53% identity with prototype T2J reovirus, and 51% identity with T1L reovirus.
The results of S1 gene phylogenetic analysis showed that MRV-HLJ/2007 belongs to the reovirus 3 serotype and is related to the other two MRV-3 strains isolated in China. Analysis of the S1 gene of MRVs belonging to different serotypes showed a strict correlation between S1 gene sequence similarity and viral serotype. Conversely, the other genome segments showed no correlation to viral serotype, suggesting that mammalian reovirus has evolved independently of serotype. This suggest that three versions of the S1 segment, corresponding to three major MRV serotypes, arose from progenitors at different times and have subsequently diverged at a rate similar to that of the other segments.
We have also analyzed the phylogenetic relationship based on the remainder of the genes. Although the phylogenetic analysis based on most genes indicated a close relationship between strains MRV-HLJ/2007 and SC-A, analyses of the M2 and S3 gene suggested that the MRV-HLJ/2007 might be derived from a strain other than SC-A, i.e., that MRV-HLJ/2007 might be a new strain circulating in China. The phylogenetic trees of individual reovirus strains differ depending on which dsRNA segments are chosen for comparison, indicating that reassortment of the MRV gene segment occurs under natural circumstances. These phylogenetic results suggest that the genes of MRV have independent ancestries [24, 25].
Our results indicate that reassortment in MRV may occur in nature and that such reassortment appears to be reflected mainly in the different S1 genotypes. The analysis based on the S2, S4, L1, L2, L3, M1, M2, and M3 genes demonstrated that both MRV-HLJ/2007 and SC-A are closely related to the MRV2Tou05 strain that was isolated from children with acute necrotizing encephalopathy. Results obtained with the S1 gene, however, showed that MRV2Tou05 is in the same clade with the 302I and 302II strain, which belong to serotype 2. These results indicate that MRV2Tou05 may be a reassortment stain that contains the genes of human and swine origin. This suggests that in vivo reassortment between serotypes 1, 2, and 3 plays a significant role in the evolution of reoviruses in nature.
In summary, the present study reports the isolation, identification, and molecular characterization of a genetically distinct reovirus serotype 3 strain. Our study suggests that MRV-HLJ/2007 might be a novel swine-infecting MRV circulating in China and that reassortment between reoviruses in nature is possible. Although swine reoviruses have not been shown to transmit efficiently within human populations and have not caused epidemics so far, these viruses may constitute a threat to humans. After further adaptation to the human host or after reassortment with other reoviruses, they may form the basis for newly emerging pandemic reoviruses in humans. These data stress the importance of annual surveillance of reoviruses in pigs, birds, and humans.
References
P. Mertens, Virus Res. 101, 3–13 (2004)
A.B. Sabin, Science 130, 1387–1389 (1959)
B. Selb, B. Weber, J. Virol. Methods 47, 15–25 (1994)
J.R. Wiener, J.A. Bartlett, W.K. Joklik, Virology 169, 293–304 (1989)
L.A. Breun, T.J. Broering, A.M. McCutcheon, S.J. Harrison, C.L. Luongo, M.L. Nibert, Virology 287, 333–348 (2001)
P. Yin, N.D. Keirstead, T.J. Broering, M.M. Arnold, J.S. Parker, M.L. Nibert, K.M. Coombs, Virol. J. 1, 6 (2004)
K.B. Chua, G. Crameri, A. Hyatt, M. Yu, M.R. Tompang, J. Rosli, J. McEachern, S. Crameri, V. Kumarasamy, B.T. Eaton, L.F. Wang, Proc. Natl. Acad. Sci. USA 104, 11424–11429 (2007)
A. Baskerville, J.B. McFerran, T. Connor, Res. Vet. Sci. 12, 172–174 (1971)
L. Kasza, Vet. Rec. 87, 681–686 (1970)
M.G. Robl, J.P. McAdaragh, C.S. Phillips, E.J. Bicknell, Vet. Med. Small Anim. Clin. 66, 903–909 (1971)
Z.Y. Zeng, W.Z. Guo, Z.W. Xu, H.Y. Liang, Z.H. Song, H.P. Yin, X. Wang, X.Y. Wang, Chin. J. Vet. Sci. 28, 799–803 (2008)
B. Blanchard, M. Kobisch, J.M. Bove, C. Saillard, Mol. Cell. Probes 10, 15–22 (1996)
C.F. Zhang, S.J. Cui, S.P. Hu, Z. Zhang, Q.Y. Guo, R. Zell, J. Virol. Methods 208–213 (2010)
G.Z. Tong, Y.J. Zhou, X.F. Hao, Z.J. Tian, T.Q. An, H.J. Qiu, Emerg. Infect. Dis. 13, 1434–1436 (2007)
R. Larochelle, A. Bielanski, P. Muller, R. Magar, J. Clin. Microbiol. 38, 4629–4632 (2000)
Y.K. Choi, S.M. Goyal, S.W. Kang, M.W. Farnham, H.S. Joo, J. Virol. Methods 102, 53–59 (2002)
T.P. Leary, J.C. Erker, M.L. Chalmers, A.T. Cruz, J.D. Wetzel, S.M. Desai, I.K. Mushahwar, T.S. Dermody, J. Clin. Microbiol. 40, 1368–1375 (2002)
T.P. Leary, J.C. Erker, M.L. Chalmers, J.D. Wetzel, S.M. Desai, I.K. Mushahwar, T.S. Dermody, J. Virol. Methods 104, 161–165 (2002)
N. Decaro, M. Campolo, C. Desario, D. Ricci, M. Camero, E. Lorusso, G. Elia, A. Lavazza, V. Martella, C. Buonavoglia, Vet. Microbiol. 109, 19–27 (2005)
H. Wulff, J.V. Lange, Bull. World Health Organ. 52, 429–436 (1975)
P.W. Lee, E.C. Hayes, W.K. Joklik, Virology 108, 134–146 (1981)
L. Song, Y. Zhou, J. He, H. Zhu, R. Huang, P. Mao, Q. Duan, Virus Genes 37, 392–399 (2008)
B.W.J. Mahy, M.H.V. Van Regenmortel, Desk Encyclopedia of Human and Medical Virology (Academic Press, Oxford, UK, 2009)
J.D. Chapell, M.I. Goral, S.E. Rodgers, C.W. de Pamphilis, T.S. Dermody, J. Virol. 68, 750–756 (1994)
E.A. Wenske, S.J. Chanock, L. Krata, B.N. Fields, J. Virol. 56, 613–616 (1985)
Acknowledgments
We thank Dr. Ulf Bech Christensen for critical reading and editing of the manuscript. The work was supported by National Hightech R&D Program (863 Program-2007AA100606) and Infectious Diseases Special Project, Minister of Health of China (2008ZX10004-001).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chaofan Zhang and Licheng Liu are contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, C., Liu, L., Wang, P. et al. A potentially novel reovirus isolated from swine in northeastern China in 2007. Virus Genes 43, 342–349 (2011). https://doi.org/10.1007/s11262-011-0642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-011-0642-4