Abstract
Cervico-vaginal mucus (CVM), the product of epithelial cells lining the uterus, cervix and vagina, is secreted to facilitate uterine lubrication and microbial clearance. Predominantly composed of water and mucins, CVM also contains high levels of immuno-active proteins such as immunoglobulin A (IgA), lactoferrin and lysozyme which protect against infection by blocking adhesion and mediating microbial killing. The repertoire of cytokines, chemokines and antimicrobial peptides is predominantly generated by the secretions of endometrial epithelial cells into the uterine lumen and concentrated in the CVM. The quantity and relative proportions of these inflammatory biomarkers are affected by diverse factors including the estrus cycle and health status of the animal and therefore potentially provide important diagnostic and prognostic indicators. We propose that measuring molecular signatures in bovine CVM could be a useful approach to identifying and monitoring genital tract pathologies in beef and dairy cows.
Similar content being viewed by others
Introduction
Cervico-vaginal mucus (CVM) represents a mixture of vaginal, cervical and uterine mucus and is composed of 92–95% of water, ions and 5–8% solid matter (Tsiligianni et al. 2001). The solid fraction is predominantly composed of mucin glycoproteins, proteoglycans, and lipids. Mucus also contains defense proteins such as secretory immunoglobulin A (IgA), lactoferrin and lysozyme (Rao et al. 1973; Tsiligianni et al. 2003). Mucin glycoproteins are responsible for the viscoelastic properties of mucus and contain proteins, sugar and sialic acid (Causey 2007; Sheehan et al. 2006; Sleigh et al. 1988). These components are highly independent and proportionally regulated; alteration in any one of which can disrupt the physical properties of mucus (Lai et al. 2009). DNA derived from the breakdown of leukocytes, epithelial cells and symbiotic bacteria in healthy animals is also present in mucus and its concentration increased in cases of infection (Sheehan et al. 2006). Live and dead microbes are found in mucus and their diversity and pathogenicity also vary according to the health status of the animal (Knudsen et al. 2015; 2016). However, the utility of CVM for prognosis and diagnosis of disease in livestock species has not been extensively explored. Here we propose that specific biomarker signatures in bovine CVM could be used to predict, before the onset of clinical symptoms, animals likely to develop genital tract pathology and to monitor their disease progress.
Mechanical role of CVM
CVM protects the reproductive tract by providing sustained lubrication and moistening of epithelial surfaces. The mucus layer represents a barrier that has been designed to prevent microbial adherence and epithelial invasion and mediate bacterial eradication (Brownlie and Hibbitt 1972; Causey 2007; Ginther 1992; Sheehan et al. 2006). In mares, failure of adequate CVM elimination through the vagina leads to its accumulation and the formation of thick and sticky plaques that facilitate bacterial colonization (Causey 2007; Sheehan et al. 2006), while, intra-luminal accumulation of CVM in the uterus decreases phagocytic activity of neutrophils leading to propagation of infection and development of postpartum uterine inflammation (Troedsson and Liu 1992).
Factors affecting CVM secretion and composition
Secretion of CVM in the genital tract is a continuous process, and the composition, quantity, physical and biochemical properties are affected by the estrus cycle and health status of cows (Lopez-Gatius et al. 1993) and women (Morales et al. 1993). Therefore, the volume and quality of CVM collected will differ significantly depending on time of sampling. Under the control of steroid hormones, mainly estrogen, CVM at ovulation is more liquid, less viscous and has a high pH than at other times of the cycle (Lopez-Gatius et al. 1993; Tsiligianni et al. 2011). Low viscosity is important to facilitate passage of spermatozoa through the mucus (Lopez-Gatius et al. 1993) while high pH promotes the viability of spermatozoa. During the luteal phase, when progesterone predominates, CVM is highly viscous and impenetrable by spermatozoa and has a low pH (Lopez-Gatius et al. 1993). Furthermore, bacteria are trapped by the luteal phase CVM and destroyed by antimicrobial peptides, and lysozymes. Genital problems such as ovarian cysts which increase progesterone secretion also increase CVM viscosity. During pregnancy, cervical mucus forms the cervical mucus plug, a highly viscous mucus that completely seals the cervix, making its penetration by microbes and contamination of the foetus less likely (Becher et al. 2009; Cortés et al. 2014).
Postpartum inflammation
Physiological inflammation
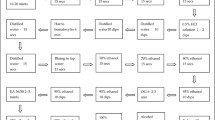
During pregnancy, the uterus is protected by a closed cervix and the thick mucus plug which contains immune cells and inflammatory mediators to protect the endometrium from infection (Lee et al. 2011) (Fig. 1). However, recent studies have demonstrated that the gravid uterus is not completely sterile but contains its own microbiome (Karstrup et al. 2017). Postpartum, physiological inflammation is essential for uterine involution which is governed by hormonal and immune mechanisms probably activated by local commensal organisms (Gabler et al. 2010; Konigsson et al. 2002) (Fig. 1). The tissue damage and stress associated with birth induces the secretion of cytokines (IL-1 and IL-6), chemokines (CXCL5, IL-8) and APPs (Carneiro et al. 2016; Horadagoda et al. 1999; Huzzey et al. 2009; Regassa and Noakes 1999; Tothova et al. 2014), all of which play a key role in tissue remodeling and repair. Haptoglobin (HP) (Sheldon et al. 2001), serum amyloid A (SAA) (Chapwanya et al. 2013) and α1- acid glycoprotein (AGP) (Williams et al. 2005) are primary positive inflammatory acute phase proteins (Tothova et al. 2014) which promote uterine repair and are concentrated in CVM (Adnane et al. 2017a). Downregulation of pro-inflammatory gene expression in healthy cows 21 days postpartum (DPP) is followed by upregulation of genes involved in tissue remodeling (Foley et al. 2015) and increased phagocytic activity of neutrophils (Jaconi et al. 1990; Martinez et al. 2014; Sayeed 2000), all of which are important for uterine repair. Dysregulation of the response to the local microbiome and/or contamination of the uterus during calving by pathogenic bacteria increases the risk of metritis which is defined as deep inflammation of the endometrium and myometrium before 21 DPP (Sheldon et al. 2006).
Early postpartum, the cervix is open allowing the mixing of uterine, cervical and vaginal secretions which form the cervico-vaginal mucus (CVM). a Healthy endometrium is protected by a thin layer of mucus composed of low number of immune cells mainly polymorphonuclear cells (PMNs) and lymphocytes, commensal microbes, DNA from degraded cells, cytokines such as interleukin 1 (IL-1) and IL-6, chemokines such as IL-8, acute phase proteins (APP) such as serum amyloid A (SAA) and haptoglobin (HP) and antimicrobial peptides (AMP) such as lactoferrin and complement proteins (Chapwanya et al. 2012; Dadarwal et al. 2017; Healy et al. 2015). These immune molecules and cells prevent microbial invasion of the uterus. b After calving, the endometrium is exposed to bacterial contamination and a deep regeneration of tissue and glands as healthy inflammation. The immune system responds by recruiting more immune cells (PMNs) to the uterus and epithelial and stromal cells increase the secretion of cytokines, chemokines and APP (SAA) to fight microbes and modulate the immune response. Furthermore, mucus secretion is increased to facilitate clearance of bacteria and their toxins (Williams et al. 2005). c If early inflammation is not resolved, sustained or elevated secretion of immune proteins leads to tissue damage, delayed involution and reproductive problems (Chapwanya et al. 2013; Kasimanickam et al. 2004; Sheldon et al. 2009b). All these mediators of inflammation and immune cells are concentrated in CVM which can be profiled for biomarkers of uterine disease (Adnane et al. 2017a; Carneiro et al. 2016; Healy et al. 2014)
Pathological inflammation
If bacteria proliferate in the uterus after calving, physiological inflammation persists and transforms to pathological and chronic inflammation leading to metritis and endometritis, respectively. High levels of inflammatory mediators are secreted to attract and activate more immune cells in the genital tract lumen (Adnane et al. 2017a; Foley et al. 2012; Sheldon et al. 2009b). Risk factors such as dystocia and metabolic disorders also lead to disrupted epithelium, exposure of the underlying stroma and contribute to the switch to pathological inflammation (Adnane et al. 2017b; Healy et al. 2015; Sheldon et al. 2009a). Persistent inflammation, which occurs in endometritis, induces tissue damage which can potentially increase bacterial and viral invasion into tissues. Furthermore, endometrial glands are rare and their secretion is switched to prostaglandin PGE2 which facilitates the multiplication of bacteria (Herath et al. 2009; Sheldon et al. 2009b). At the same time, CVM secretion increases and its composition changes to became more viscous (Williams et al. 2005). As a result, inflammatory mediators, immune cells and bacteria become trapped in the CVM and therefore can potentially be used to assess the microbial and immune status.
Informative molecular signatures in CVM
CVM from cows with clinical endometritis was recently found to contains over 3 times the amount of total protein compared to CVM from healthy cows 21 DPP (Adnane et al. 2017a). In addition to mucins, the total protein is composed of degraded membrane and cytoplasmic proteins of dead epithelial and immune cells as well as bacterial cell walls (Sheehan et al. 2006; Sleigh et al. 1988). Any of these molecules may represent potential markers to detect uterine problems soon after calving, before the onset of clinical symptoms, as the current diagnostic methods of uterine disease are only employed after the appearance of symptoms (21 DPP),
Mucins
Mucin fibers are crosslinked, bundled and entangled protein fibers of 10–40 MDa in size and are usually glycosylated via proline, threonine, and/or serine residues (Carlstedt and Sheehan 1984). Human respiratory tract mucins are the best described and are negatively charged since mucin glycoproteins are rich in sialic acid and sulfate which increases the rigidity of the polymer (Shogren et al. 1989). Secretion of mucins in respiratory mucus of different species is influenced by multiple factors including the presence of pathogens, inflammatory biomarkers and toxins (Rose and Voynow 2006; Thai et al. 2008). Microbial associated molecular patterns (MAMPs) can activate epithelial cell surface receptors (i.e. Toll-like receptors) leading to NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation and upregulation of MUC2 and/or MUC5AC transcription (Rose and Voynow 2006). Cytokines and chemokines such as TNF-α, IL-1β, IL-8 and IL-13 upregulate MUC5AC while, IL-16 and IL-17 upregulate MUC5AC and MUC5B in human and animal epithelial cells (Rose and Voynow 2006; Voynow and Rubin 2009). The cell-associated MUC1 is thought to play a role as a receptor for bacterial components and lipopolysaccharides (LPS) have been shown to induce the expression of MUC1 in bovine endometrial epithelial cells (Sando et al. 2009). However, MUC1 binding of flagellin from Pseudomonas aeruginosa has been shown to inhibit binding to TLR-5 and IL-8 secretion, thereby facilitating persistence of the bacteria. In a similar manner, MUC1 prevents pathogens from binding to the endometrium by inhibiting cell-to-cell binding. MUC1 has also been shown to be associated with infertility as it interferes with the implantation of the embryo in the epithelial endometrium in ruminants (Johnson et al. 2001) and humans (Wesseling et al. 1995). Therefore, MUC1 is naturally removed from local sites of interaction between trophoblast and endometrial epithelium in different species (Johnson et al. 2001; Meseguer et al. 2001). Measuring levels of MUC1 in CVM may be relevant to early diagnosis of fertility problems and recurrent miscarriage. As secreted mucin, MUC2 plays an anti-inflammatory role by preventing colonic epithelial inflammation in mice (Van der Sluis et al. 2006). However, properties of secreted mucins are not well understood (Dekker et al. 2002; Hoorens et al. 2011). MUC4 is reported to be involved in the activation of the receptor tyrosine-protein kinase ErbB2, an epidermal growth factor receptor and its overexpression is correlated with the occurrence of various human cancers (Ramsauer et al. 2006; Voynow and Rubin 2009). MUC5AC concentrations in CVM may be diagnostic of endometrial gland abnormalities because upregulated expression of MUC5AC in human airway mucus occurs through the activation of NF-κB pathway when prostaglandin PGE2 secretion is increased (Gray et al. 2004). PGE2 is important for Escherichia coli (E. coli) multiplication in the uterus (Sheldon et al. 2009b). Furthermore, IL-8 and TNF-α are known to upregulate the expression of MUC5AC by increasing the stability of its mRNA. Many cytokines implicated in postpartum endometrial inflammation stimulate mucin secretion (Carneiro et al. 2016; Healy et al. 2015; Sheldon et al. 2009a) and therefore measuring mucins in CVM is potentially informative in terms of pathological inflammation, infertility and possibly other genital tract diseases.
Cytokines
Some cytokines (e.g. IL-6) are secreted directly into the endometrial lumen while TNF-α, IL-1 and IL-8 are concentrated in uterine mucus after infiltrating through uterine wall (Carneiro et al. 2016; Oliveira et al. 2012). Analysis of these molecules in CVM has been used to detect lower genital tract pathologies (Van Raemdonck et al. 2014; Zegels et al. 2010) in human (Table 1). Cytokines have previously been measured in mucus collected by uterine washings. TNF-α levels were shown to be elevated at 22 DPP in uterine mucus of cows with subclinical endometritis compared to healthy cows (Brodzki et al. 2015a). Likewise, uterine mucus from cows diagnosed with pyometra contains elevated levels of TNF-α at 70–90 DPP (Brodzki et al. 2015b). However, collecting mucus using uterine lavage may underestimate the true level of biomarkers as uterine secretions are diluted using this approach. Furthermore, Cheong et al. (2011) reported a decrease in pregnancy rate at first insemination in primiparous cows following uterine lavage, implying that intra-uterine fluid infusion may initiate a level of inflammation (Cheong et al. 2011; Kasimanickam et al. 2005) which may not be desirable. Other studies reported that inflammatory markers were differentially concentrated in CVM in cows following dystocia (Cronin et al. 2015; Healy et al. 2014; 2015) (Table 1), while we described a method for successfully measuring inflammatory cytokines in CVM collected directly from early postpartum cows (Adnane et al. 2017a) (Table 1).
IL-1 is the key mediator of uterine inflammation that is secreted as a result of tissue damage associated with parturition (Adnane et al. 2017a; Healy et al. 2014) and uterine involution. Measuring IL-1 levels in CVM could be useful to monitor uterine health status as cows with clinical endometritis have persistent increased level of IL-1β in uterine mucus from calving to the eighth week post calving (Kim et al. 2014). However, CVM is easier to collect than uterine mucus. In our previous study, we found that IL-1β is highly elevated in cows with clinical endometritis early postpartum (Table 1) (Adnane et al. 2017a). We identified IL-1β levels in CVM at 7 days postpartum to be a predictor of cows likely to subsequently develop endometritis 3 weeks after calving. Similarly, IL-6, measured in uterine mucus (Brodzki et al. 2015a; c) and CVM (Adnane et al. 2017a; Healy et al. 2014; 2015) detects clinical and subclinical uterine inflammation and tissue damage associated with dystocia (Table 1). Endometritis is associated with increased expression of IL8 in the endometrium 2 weeks after calving, and intrauterine infusion of exogenous IL-8 reproduces the disease (Chapwanya et al. 2009; Zerbe et al. 2003). IL-8 accumulates in CVM where as a chemokine, it attracts neutrophils to the uterine lumen to eliminate bacteria (Abou Mossallam et al. 2015). IL-8 is an excellent indicator of inflammation because it increases 10- to 100-fold in response to pro-inflammatory cytokines, bacterial or viral products, or cellular stress (Hoffmann et al. 2002). During first week postpartum, IL-8 was differentially secreted in bovine uterine mucus in the case of dystocia (Table 1) (Cronin et al. 2015; Healy et al. 2014) and clinical endometritis (Adnane et al. 2017a). Many other studies about gene expression of cytokines in the endometrial environment have been reported (Wagener et al. 2017).
Acute phase proteins and complement components
APPs are highly responsive proteins, secreted by the liver in response to injury or infection in an effort to restore homeostasis. Extra-hepatic sources of APPs have also been identified, including in endometrial cells (Chapwanya et al. 2013). They are often measured in serum and proposed as diagnostic biomarkers (Brodzki et al. 2015a; b; c; Kim et al. 2014). However, their specificity for uterine disease is likely to be affected by the occurrence of additional diseases including mastitis. APPs such as HP, SAA and AGP in CVM are useful to monitor uterine inflammation postpartum if they are measured during the first week postpartum (Adnane et al. 2017a) (Table 1). SAA is secreted 24-48 h after infection under stimulation of IL-1 and/or TNF-α (Petersen et al. 2004; Tothova et al. 2014) and has high opsonization activity to gram-negative bacteria (Hari-Dass et al. 2005; Shah et al. 2006). SAA is an informative predictor of uterine health status as it is produced locally by the endometrial epithelial cells and its gene expression and protein secretion are increased in response to E. coli infection (Chapwanya et al. 2013). Cows that developed subclinical endometritis had lower level of SAA in the uterine mucus at 5 days postpartum compared to healthy cows (Brodzki et al. 2015a). It seems that SAA plays the role of inflammatory regulator to prevent tissue damage induced by severe and prolonged inflammation in the endometrium. HP is mainly secreted by erythrocytes and may penetrate to the uterine lumen from the blood circulation (Brodzki et al. 2015c). Levels of HP in uterine mucus are elevated in cows with subclinical endometritis and pyometra late postpartum (60–90 DPP) (Brodzki et al. 2015b; c) (Table 1). We found that healthy cows had higher levels of SAA and lower levels of HP in CVM, compared to cows with clinical endometritis, diagnosed at 21 DPP (Adnane et al. 2017a) (Table 1). Systemic levels of HP have been reported not to be affected by the health status of the uterus (Yasui et al. 2014). AGP is another interesting APP and its concentration increases early postpartum as part of the normal process of tissue repair and wound healing, and decreases gradually during uterine involution to regain its normal concentration in serum at day 21 postpartum (Regassa and Noakes 1999; Sheldon et al. 2001). However, its secretion is affected by the health status of the uterus and cows with fetid vaginal discharge or infected with E. coli have high systemic levels of AGP at 21 and 28 DPP (Williams et al. 2005). Levels of complement components C3 and C5b were shown to be differentially secreted in CVM of cows infected with Trichomonas foetus or endometritis (Adnane et al. 2017a; Kania et al. 2001)
Antimicrobial peptides
Members of the S100 family chelate calcium and therefore regulate many cellular processes including microbial viability (Corbin et al. 2008; Ibrahim et al. 2016). Expression of S100A9 and S100A8 is highly upregulated in endometrial tissue of cows challenged with severe negative energy balance, and in endometrial cervical tissue of humans affected with uterine and cervical cancers (Kostakis et al. 2010; Wathes et al. 2009). Interestingly, S100A9 are differentially expressed at the endometrial tissue between cows with cytological endometritis and healthy group at 7 and 21 DPP (Foley et al. 2015). Furthermore, S100A8 and S100A9 secretion is not affected by estrus cycle which make them stable makers at any time of sampling (Ibrahim et al. 2016). Measurement of S100 s levels in CVM or uterine mucus in postpartum cows may provide a useful diagnostic tool. Lactoferrin is produced mainly by epithelial cells and is considered to be anti-inflammatory and immunomodulatory with particular antibacterial properties (Cooper et al. 2014; Rao et al. 1973). It is widely distributed in body fluids (Metz-Boutigue et al. 1984). Although there are no available data on lactoferrin measurement in CVM, we believe that it represents a potentially informative marker for postpartum uterine inflammation, since it is known to suppress LPS-induced endometritis by binding to LPS and blocking its action to activate inflammation through NFkB pathway (Latorre et al. 2012; Li et al. 2015). Defensins are short cationic peptides with potent immunoregulatory and antimicrobial activity secreted by epithelial cells. They provide protection to all mucosal surfaces including the intestine, the lung and the reproductive tract (Davies et al. 2008; Sheehan et al. 2006; Wilson et al. 2016). A major expansion in the beta defensin gene repertoire has been discovered in the bovine genome (Meade et al. 2014) and several genes encoding for β-defensin were highly expressed at the endometrial tissue of cows with clinical endometritis 21 DPP (Foley et al. 2015). The expression signature of defensins in CVM is likely to be a useful indicator of local health and infection. The microbial diversity of CVM might also be of prognostic or diagnostic value.
Conclusion
Here we reviewed the measurement of inflammatory biomarkers in CVM early postpartum as an alternative method for the prognosis of genital tract pathology in cows and other species. CVM molecular signatures are specific to uterine health status may also reflect local microbiome diversity. They provide a convenient, cost effective and welfare-friendly method for timely detection of uterine inflammation in order to reduce the impact of endometritis on dairy cow production. CVM therefore is a potentially valuable resource to investigate the diversity of bacteria in cows with uterine disease as many of these bacteria are trapped by the mucus structure.
References
Abou Mossallam AA, El Nahas SM, Mahfouz ER, Osman NM (2015) Characterization of buffalo interleukin 8 (IL-8) and its expression in endometritis. J Genet Eng Biotechnol 13:71–77. https://doi.org/10.1016/j.jgeb.2014.12.007
Adnane M, Chapwanya A, Kaidi R, Meade KG, O'Farrelly C (2017a) Profiling inflammatory biomarkers in cervico-vaginal mucus (CVM) postpartum: potential early indicators of bovine clinical endometritis? Theriogenology 103:117–122. https://doi.org/10.1016/j.theriogenology.2017.07.039
Adnane M, Kaidi R, Hanzen C, England GCW (2017b) Risk factors of clinical and subclinical endometritis in cattle: a review. Turk J Vet Anim Sci 41:1–11. https://doi.org/10.3906/vet-1603-63
Becher N, Adams Waldorf K, Hein M, Uldbjerg N (2009) The cervical mucus plug: structured review of the literature. Acta Obstet Gynecol Scand 88:502–513. https://doi.org/10.1080/00016340902852898
Brodzki P, Kostro K, Brodzki A, Wawron W, Marczuk J, Kurek L (2015a) Inflammatory cytokines and acute-phase proteins concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology 84:11–18. https://doi.org/10.1016/j.theriogenology.2015.02.006
Brodzki P, Kostro K, Brodzki A, Zietek J (2015b) The concentrations of inflammatory cytokines and acute-phase proteins in the peripheral blood and uterine washings in cows with pyometra. Reproduction in domestic animals =. Zuchthygiene 50:417–422. https://doi.org/10.1111/rda.12507
Brodzki P, Kostro K, Krakowski L, Marczuk J (2015c) Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet Res Commun 39:143–149. https://doi.org/10.1007/s11259-015-9635-4
Brownlie J, Hibbitt KG (1972) Antimicrobial proteins isolated from bovine cervical mucus. J Reprod Fertil 29:337–347
Carlstedt I, Sheehan JK (1984) Macromolecular properties and polymeric structure of mucus glycoproteins. CIBA Found Symp 109:157–172
Carneiro LC, Cronin JG, Sheldon IM (2016) Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod Biol 16:1–7. https://doi.org/10.1016/j.repbio.2015.12.002
Causey RC (2007) Mucus and the mare: how little we know. Theriogenology 68:386–394. https://doi.org/10.1016/j.theriogenology.2007.04.011
Chapwanya A, Meade KG, Doherty ML, Callanan JJ, Mee JF, O'Farrelly C (2009) Histopathological and molecular evaluation of Holstein-Friesian cows postpartum: toward an improved understanding of uterine innate immunity. Theriogenology 71:1396–1407. https://doi.org/10.1016/j.theriogenology.2009.01.006
Chapwanya A, Meade KG, Foley C, Narciandi F, Evans ACO, Doherty ML, Callanan JJ, O'Farrelly C (2012) The postpartum endometrial inflammatory response: a normal physiological event with potential implications for bovine fertility. Reprod Fertil Dev 24:1028–1039. https://doi.org/10.1071/RD11153
Chapwanya A, Meade KG, Doherty ML, Callanan JJ, O'Farrelly C (2013) Endometrial epithelial cells are potent producers of tracheal antimicrobial peptide and serum amyloid A3 gene expression in response to E. Coli stimulation. Vet Immunol Immunopathol 151:157–162. https://doi.org/10.1016/j.vetimm.2012.09.042
Cheong SH, Nydam DV, Galvao KN, Crosier BM, Gilbert RO (2011) Cow-level and herd-level risk factors for subclinical endometritis in lactating Holstein cows. J Dairy Sci 94:762–770. https://doi.org/10.3168/jds.2010-3439
Cooper C, Nonnecke E, Lönnerdal B, Murray J (2014) The lactoferrin receptor may mediate the reduction of eosinophils in the duodenum of pigs consuming milk containing recombinant human lactoferrin. Biometals 27:1031–1038. https://doi.org/10.1007/s10534-014-9778-8
Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965. https://doi.org/10.1126/science.1152449
Cortés ME, González F, Vigil P (2014) Crystallization of bovine cervical mucus at Oestrus: an update. Revista de Medicina Veterinaria:103–116
Cronin JG, Hodges R, Pedersen S, Sheldon IM (2015) Enzyme linked immunosorbent assay for quantification of bovine interleukin-8 to study infection and immunity in the female genital tract. Am J Reprod Immunol 73:372–382. https://doi.org/10.1111/aji.12344
Dadarwal D, Palmer C, Griebel P (2017) Mucosal immunity of the postpartum bovine genital tract. Theriogenology 104:62–71. https://doi.org/10.1016/j.theriogenology.2017.08.010
Davies D, Meade KG, Herath S, Eckersall PD, Gonzalez D, White JO, Conlan RS, O'Farrelly C, Sheldon IM (2008) Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod Biol Endocrinol 6:53. https://doi.org/10.1186/1477-7827-6-53
Dekker J, Rossen JW, Buller HA, Einerhand AW (2002) The MUC family: an obituary. Trends Biochem Sci 27:126–131. https://doi.org/10.1016/s0968-0004(01)02052-7
Foley C, Chapwanya A, Creevey CJ, Narciandi F, Morris D, Kenny EM, Cormican P, Callanan JJ, O’Farrelly C, Meade KG (2012) Global endometrial transcriptomic profiling: transient immune activation precedes tissue proliferation and repair in healthy beef cows. BMC Genomics 13:489. https://doi.org/10.1186/1471-2164-13-489
Foley C, Chapwanya A, Callanan JJ, Whiston R, Miranda-CasoLuengo R, Lu J, Meijer WG, Lynn DJ, O’ Farrelly C, Meade KG (2015) Integrated analysis of the local and systemic changes preceding the development of post-partum cytological endometritis. BMC Genomics 16:811. https://doi.org/10.1186/s12864-015-1967-5
Gabler C, Fischer C, Drillich M, Einspanier R, Heuwieser W (2010) Time-dependent mRNA expression of selected pro-inflammatory factors in the endometrium of primiparous cows postpartum. Reprod Biol Endocrinol 8:152. https://doi.org/10.1186/1477-7827-8-152
Ginther OJ (1992) Reproductive biology of the mare: basic and applied aspects. Equiservices, Cross Plains
Gray T, Nettesheim P, Loftin C, Koo JS, Bonner J, Peddada S, Langenbach R (2004) Interleukin-1beta-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase a signaling. Mol Pharmacol 66:337–346. https://doi.org/10.1124/mol.66.2.337
Hari-Dass R, Shah C, Meyer DJ, Raynes JG (2005) Serum amyloid a protein binds to outer membrane protein a of gram-negative bacteria. J Biol Chem 280:18562–18567. https://doi.org/10.1074/jbc.M500490200
Healy LL, Cronin JG, Sheldon IM (2014) Endometrial cells sense and react to tissue damage during infection of the bovine endometrium via interleukin 1. Sci Rep 4:7060. https://doi.org/10.1038/srep07060
Healy LL, Cronin JG, Sheldon IM (2015) Polarized epithelial cells secrete interleukin 6 apically in the bovine endometrium. Biol Reprod 92:151. https://doi.org/10.1095/biolreprod.115.127936
Herath S, Lilly ST, Fischer DP, Williams EJ, Dobson H, Bryant CE, Sheldon IM (2009) Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2alpha to prostaglandin E2 in bovine endometrium. Endocrinology 150:1912–1920. https://doi.org/10.1210/en.2008-1379
Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M (2002) Multiple control of interleukin-8 gene expression. J Leukoc Biol 72:847–855
Hoorens PR, Rinaldi M, Li RW, Goddeeris B, Claerebout E, Vercruysse J, Geldhof P (2011) Genome wide analysis of the bovine mucin genes and their gastrointestinal transcription profile. BMC Genomics 12:140. https://doi.org/10.1186/1471-2164-12-140
Horadagoda NU, Knox KM, Gibbs HA, Reid SW, Horadagoda A, Edwards SE, Eckersall PD (1999) Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet Rec 144:437–441
Huzzey JM, Duffield TF, LeBlanc SJ, Veira DM, Weary DM, von Keyserlingk MA (2009) Short communication: Haptoglobin as an early indicator of metritis. J Dairy Sci 92:621–625. https://doi.org/10.3168/jds.2008-1526
Ibrahim M, Peter S, Gartner MA, Michel G, Jung M, Einspanier R, Gabler C (2016) Increased mRNA expression of selected antimicrobial peptides around ovulation and during inflammatory processes in the bovine endometrium postpartum. Theriogenology 86:2040–2053. https://doi.org/10.1016/j.theriogenology.2016.06.022
Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjogren M, Stendahl O (1990) Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol 110:1555–1564
Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC (2001) Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod 65:820–828
Kania SA, Reed SL, Thomford JW, BonDurant RH, Hirata K, Corbeil RR, North MJ, Corbeil LB (2001) Degradation of bovine complement C3 by trichomonad extracellular proteinase. Vet Immunol Immunopathol 78:83–96
Karstrup CC, Klitgaard K, Jensen TK, Agerholm JS, Pedersen HG (2017) Presence of bacteria in the endometrium and placentomes of pregnant cows. Theriogenology 99:41–47. https://doi.org/10.1016/j.theriogenology.2017.05.013
Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH (2004) Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology 62:9–23. https://doi.org/10.1016/j.theriogenology.2003.03.001
Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH (2005) A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can Vet J 46:255–259
Kim IH, Kang HG, Jeong JK, Hur TY, Jung YH (2014) Inflammatory cytokine concentrations in uterine flush and serum samples from dairy cows with clinical or subclinical endometritis. Theriogenology 82:427–432. https://doi.org/10.1016/j.theriogenology.2014.04.022
Knudsen LR, Karstrup CC, Pedersen HG, Agerholm JS, Jensen TK, Klitgaard K (2015) Revisiting bovine pyometra--new insights into the disease using a culture-independent deep sequencing approach. Vet Microbiol 175:319–324. https://doi.org/10.1016/j.vetmic.2014.12.006
Knudsen LR et al (2016) An investigation of the microbiota in uterine flush samples and endometrial biopsies from dairy cows during the first 7 weeks postpartum. Theriogenology 86:642–650. https://doi.org/10.1016/j.theriogenology.2016.02.016
Konigsson K, Gustafsson H, Kindahl H (2002) 15-ketodihydro- PGF2alpha, progesterone and uterine involution in primiparous cows with induced retained placenta and post-partal endometritis treated with oxytetracycline and flunixin. Reprod Dom Anim 37:43–51
Kostakis ID, Cholidou KG, Kallianidis K, Perrea D, Antsaklis A (2010) The role of calprotectin in obstetrics and gynecology. Eur J Obstet Gynecol Reprod Biol 151:3–9. https://doi.org/10.1016/j.ejogrb.2010.03.006
Lai SK, Wang YY, Wirtz D, Hanes J (2009) Micro- and macrorheology of mucus. Adv Drug Deliv Rev 61:86–100. https://doi.org/10.1016/j.addr.2008.09.012
Latorre D, Berlutti F, Valenti P, Gessani S, Puddu P (2012) LF immunomodulatory strategies: mastering bacterial endotoxin1This article is part of a special issue entitled Lactoferrin and has undergone the Journal's usual peer review process. Biochem Cell Biol 90:269–278. https://doi.org/10.1139/o11-059
Lee DC, Hassan SS, Romero R, Tarca AL, Bhatti G, Gervasi MT, Caruso JA, Stemmer PM, Kim CJ, Hansen LK, Becher N, Uldbjerg N (2011) Protein profiling underscores immunological functions of uterine cervical mucus plug in human pregnancy. J Proteome 74:817–828. https://doi.org/10.1016/j.jprot.2011.02.025
Li W, Fu K, Lv X, Wang Y, Wang J, Li H, Tian W, Cao R (2015) Lactoferrin suppresses lipopolysaccharide-induced endometritis in mice via down-regulation of the NF-kappaB pathway. Int Immunopharmacol 28:695–699. https://doi.org/10.1016/j.intimp.2015.07.040
Lopez-Gatius F, Miro J, Sebastian I, Ibarz A, Labernia J (1993) Rheological properties of the anterior vaginal fluid from superovulated dairy heifers at estrus. Theriogenology 40:167–180
Luo L et al (2000) Interleukin-8 levels and granulocyte counts in cervical mucus during pregnancy. Am J Reprod Immunol 43:78–84
Martinez N, Sinedino LDP, Bisinotto RS, Ribeiro ES, Gomes GC, Lima FS, Greco LF, Risco CA, Galvão KN, Taylor-Rodriguez D, Driver JP, Thatcher WW, Santos JEP (2014) Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. J Dairy Sci 97:874–887. https://doi.org/10.3168/jds.2013-7408
Meade KG, Cormican P, Narciandi F, Lloyd A, O'Farrelly C (2014) Bovine beta-defensin gene family: opportunities to improve animal health? Physiol Genomics 46:17–28. https://doi.org/10.1152/physiolgenomics.00085.2013
Meseguer M, Aplin JD, Caballero-Campo P, O’Connor JE, Martín JC, Remohí J́, Pellicer A, Simón C (2001) Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod 64:590–601
Metz-Boutigue MH et al (1984) Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem 145:659–676
Morales P, Roco M, Vigil P (1993) Human cervical mucus: relationship between biochemical characteristics and ability to allow migration of spermatozoa. Hum Reprod 8:78–83
Oliveira LJ, Barreto RS, Perecin F, Mansouri-Attia N, Pereira FT, Meirelles FV (2012) Modulation of maternal immune system during pregnancy in the cow. Reproduction in domestic animals =. Zuchthygiene 47(Suppl 4):384–393. https://doi.org/10.1111/j.1439-0531.2012.02102.x
Petersen HH, Nielsen JP, Heegaard PM (2004) Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res 35:163–187. https://doi.org/10.1051/vetres:2004002
Ramsauer VP, Pino V, Farooq A, Carothers Carraway CA, Salas PJ, Carraway KL (2006) Muc4-ErbB2 complex formation and signaling in polarized CACO-2 epithelial cells indicate that Muc4 acts as an unorthodox ligand for ErbB2. Mol Biol Cell 17:2931–2941. https://doi.org/10.1091/mbc.e05-09-0895
Rao KS, Roberts TK, Masson PL, Heremans JF (1973) Lactoferrin, a major soluble protein of bovine oestrous cervical mucus. J Reprod Fertil 32:89–92
Regassa F, Noakes DE (1999) Acute phase protein response of ewes and the release of PGFM in relation to uterine involution and the presence of intrauterine bacteria. Vet Rec 144:502–506. https://doi.org/10.1136/vr.144.18.502
Rose MC, Voynow JA (2006) Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 86:245–278. https://doi.org/10.1152/physrev.00010.2005
Sakai M, Ishiyama A, Tabata M, Sasaki Y, Yoneda S, Shiozaki A, Saito S (2004) Relationship between cervical mucus interleukin-8 concentrations and vaginal bacteria in pregnancy. Am J Reprod Immunol 52:106–112. https://doi.org/10.1111/j.1600-0897.2004.00203.x
Sando L, Pearson R, Gray C, Parker P, Hawken R, Thomson PC, Meadows JRS, Kongsuwan K, Smith S, Tellam RL (2009) Bovine Muc1 is a highly polymorphic gene encoding an extensively glycosylated mucin that binds bacteria. J Dairy Sci 92:5276–5291. https://doi.org/10.3168/jds.2009-2216
Sayeed MM (2000) Exuberant ca(2+) signaling in neutrophils: a cause for concern. News Physiol Sci 15:130–136
Shah C, Hari-Dass R, Raynes JG (2006) Serum amyloid a is an innate immune opsonin for gram-negative bacteria. Blood 108:1751–1757. https://doi.org/10.1182/blood-2005-11-011932
Sheehan JK, Kesimer M, Pickles R (2006) Innate immunity and mucus structure and function. Novartis found Symp 279:155-166; discussion 167-159, 216-159
Sheldon IM, Noakes DE, Rycroft A, Dobson H (2001) Acute phase protein responses to uterine bacterial contamination in cattle after calving. Vet Rec 148:172–175. https://doi.org/10.1136/vr.148.6.172
Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO (2006) Defining postpartum uterine disease in cattle. Theriogenology 65:1516–1530. https://doi.org/10.1016/j.theriogenology.2005.08.021
Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ (2009a) Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod 81:1025–1032. https://doi.org/10.1095/biolreprod.109.077370
Sheldon IM, Price SB, Cronin J, Gilbert RO, Gadsby JE (2009b) Mechanisms of infertility associated with clinical and subclinical endometritis in high producing dairy cattle. Reproduction in domestic animals =. Zuchthygiene 44(Suppl 3):1–9. https://doi.org/10.1111/j.1439-0531.2009.01465.x
Shogren R, Gerken TA, Jentoft N (1989) Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry 28:5525–5536
Sleigh MA, Blake JR, Liron N (1988) The propulsion of mucus by cilia. Am Rev Respir Dis 137:726–741. https://doi.org/10.1164/ajrccm/137.3.726
Thai P, Loukoianov A, Wachi S, Wu R (2008) Regulation of airway mucin gene expression. Annu Rev Physiol 70:405–429. https://doi.org/10.1146/annurev.physiol.70.113006.100441
Tothova C, Nagy O, Seidel H, Kovac G (2014) Acute phase proteins and their use in the diagnosis of diseases in ruminants: a reviw. Vet MedCzech 59:163–180
Troedsson MH, Liu IK (1992) Measurement of total volume and protein concentration of intrauterine secretion after intrauterine inoculation of bacteria in mares that were either resistant or susceptible to chronic uterine infection. Am J Vet Res 53:1641–1644
Tsiligianni T, Karagiannidis A, Brikas P, Saratsis P (2001) Chemical properties of bovine cervical mucus during normal estrus and estrus induced by progesterone and/or PGF2α. Theriogenology 56:41–50. https://doi.org/10.1016/S0093-691X(01)00541-6
Tsiligianni T, Karagiannidis A, Saratsis P, Brikas P (2003) Enzyme activity in bovine cervical mucus during spontaneous and induced estrus. Can J Vet Res 67:189–193
Tsiligianni T, Amiridis GS, Dovolou E, Menegatos I, Chadio S, Rizos D, Gutierrez–Adan A (2011) Association between physical properties of cervical mucus and ovulation rate in superovulated cows. Can J Vet Res 75:248–253
Van der Sluis M et al (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131:117–129. https://doi.org/10.1053/j.gastro.2006.04.020
Van Raemdonck GA, Tjalma WA, Coen EP, Depuydt CE, Van Ostade XW (2014) Identification of protein biomarkers for cervical cancer using human cervicovaginal fluid. PLoS One 9:e106488. https://doi.org/10.1371/journal.pone.0106488
Voynow JA, Rubin BK (2009) Mucins, mucus, and sputum. Chest 135:505–512. https://doi.org/10.1378/chest.08-0412
Wagener K, Gabler C, Drillich M (2017) A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology 94:21–30. https://doi.org/10.1016/j.theriogenology.2017.02.005
Wathes DC, Cheng Z, Chowdhury W, Fenwick MA, Fitzpatrick R, Morris DG, Patton J, Murphy JJ (2009) Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol Genomics 39:1–13. https://doi.org/10.1152/physiolgenomics.00064.2009
Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J (1995) Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 129:255–265
Williams EJ, Fischer DP, Pfeiffer DU, England GC, Noakes DE, Dobson H, Sheldon IM (2005) Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63:102–117. https://doi.org/10.1016/j.theriogenology.2004.03.017
Wilson SS, Wiens ME, Holly MK, Smith JG (2016) Defensins at the mucosal surface: latest insights into Defensin-virus interactions. J Virol 90:5216–5218. https://doi.org/10.1128/JVI.00904-15
Yasui T, McCann K, Gilbert RO, Nydam DV, Overton TR (2014) Associations of cytological endometritis with energy metabolism and inflammation during the periparturient period and early lactation in dairy cows. J Dairy Sci 97:2763–2770. https://doi.org/10.3168/jds.2013-7322
Zegels G, Van Raemdonck GA, Tjalma WA, Van Ostade XW (2010) Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci 8:63. https://doi.org/10.1186/1477-5956-8-63
Zerbe H, Schuberth HJ, Engelke F, Frank J, Klug E, Leibold W (2003) Development and comparison of in vivo and in vitro models for endometritis in cows and mares. Theriogenology 60:209–223
Funding
This review and MA internship were supported by the following grants: SFI Investigators Programme 2013 12/Ia/1667, HRB Health Research Award 2012 HRA_POR/2012/37 and Dept Agriculture FIRM/RSF/CoFoRD 2013–2016: ENRICH. The open access was supported by the grant: Dept Agriculture FIRM/RSF/CoFoRD 2011-2015.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to this review.
Corresponding author
Ethics declarations
Ethics approval
No ethical approval was required.
Availability of data and materials
No data are generated during this review.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Adnane, M., Meade, K.G. & O’Farrelly, C. Cervico-vaginal mucus (CVM) – an accessible source of immunologically informative biomolecules. Vet Res Commun 42, 255–263 (2018). https://doi.org/10.1007/s11259-018-9734-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-018-9734-0