Abstract
Purpose
Calcium-sensing receptor (CASR) influences the expression pattern of multiple genes in renal tubular epithelial cells. The objective of this inquiry was to explore the molecular mechanisms of CASR in renal tubular epithelial cells and nephrolithiasis.
Methods
HK-2 cells were transfected with lentiviruses carrying either CASR (named CASR) or an empty vector negative control (named NC), as well as shRNA intended to target CASR (named shCASR) or its corresponding negative control (named shNC). CCK-8 assay was used to detect the effect of CASR on the proliferation of HK-2 cells. RNA-Sequencing was applied to explore potential pathways regulated by CASR in HK-2 cells.
Results
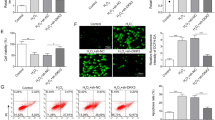
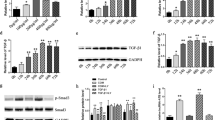
PCR and western blot results showed that CASR expression was significantly increased in CASR cells and was decreased in shCASR cells when compared to their corresponding negative control, respectively. CCK-8 assay revealed that CASR inhibited the proliferation of HK-2 cells. RNA-Sequencing results suggested that the shCASR HK-2 cells exhibited a significant up-regulation of 345 genes and a down-regulation of 366 genes. These differentially expressed genes (DEGs) were related to cell apoptosis and cell development. In CASR HK-2 cells, 1103 DEGs primarily functioned in mitochondrial energy metabolism, and amino acid metabolism. With the Venn diagram, 4 DEGs (Clorf116, ENPP3, IL20RB, and CLDN2) were selected as the hub genes regulated by CASR. Enrichment analysis revealed that these hub genes were involved in cell–cell junction, and epithelial cell development.
Conclusions
In summary, our investigation has the potential to offer novel perspectives on CASR regulating cell–cell junction in HK-2 cells.







Similar content being viewed by others
Data availability
All data experimental data are available upon request.
References
Lieske JC, Toback FG (1996) Interaction of urinary crystals with renal epithelial cells in the pathogenesis of nephrolithiasis. Semin Nephrol 16:458–473
Ding T, Zhao T, Li Y, Liu Z, Ding J, Ji B, Wang Y, Guo Z (2021) Vitexin exerts protective effects against calcium oxalate crystal-induced kidney pyroptosis in vivo and in vitro. Phytomedicine 86:153562
Riccardi D, Valenti G (2016) Localization and function of the renal calcium-sensing receptor. Nat Rev Nephrol 12:414–425
Riccardi D, Brown EM (2010) Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298:F485-499
Vezzoli G, Terranegra A, Soldati L (2012) Calcium-sensing receptor gene polymorphisms in patients with calcium nephrolithiasis. Curr Opin Nephrol Hypertens 21:355–361
Vezzoli G, Macrina L, Magni G, Arcidiacono T (2019) Calcium-sensing receptor: evidence and hypothesis for its role in nephrolithiasis. Urolithiasis 47:23–33
Li X, Chen S, Feng D, Fu Y, Wu H, Lu J, Bao J (2021) Calcium-sensing receptor promotes calcium oxalate crystal adhesion and renal injury in Wistar rats by promoting ROS production and subsequent regulation of PS ectropion, OPN, KIM-1, and ERK expression. Ren Fail 43:465–476
Ibeh CL, Yiu AJ, Kanaras YL, Paal E, Birnbaumer L, Jose PA, Bandyopadhyay BC (2019) Evidence for a regulated Ca(2+) entry in proximal tubular cells and its implication in calcium stone formation. J Cell Sci 132:jcs225268
Renkema KY, Bindels RJ, Hoenderop JG (2011) Role of the calcium-sensing receptor in reducing the risk for calcium stones. Clin J Am Soc Nephrol 6:2076–2082
Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG (2009) The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20:1705–1713
Vezzoli G, Terranegra A, Rainone F, Arcidiacono T, Cozzolino M, Aloia A, Dogliotti E, Cusi D, Soldati L (2011) Calcium-sensing receptor and calcium kidney stones. J Transl Med 9:201
Zhao J, Wu Y, Zhou K, Huang M, Sun Y, Kang J, Su Q, Zhao Y, Liu Q, Li C (2023) Ferroptosis in calcium oxalate kidney stone formation and the possible regulatory mechanism of ANKRD1. Biochim Biophys Acta Mol Cell Res 1870:119452
Dong C, Song C, He Z, Song Q, Song T, Liu J, Xiong Y, Su X, Zhou J, Yang S, Liao W (2023) Protective efficacy of Schizandrin B on ameliorating nephrolithiasis via regulating GSK3β/Nrf2 signaling-mediated ferroptosis in vivo and in vitro. Int Immunopharmacol 117:110042
He Z, Liao W, Song Q, Li B, Liu J, Xiong Y, Song C, Yang S (2021) Role of ferroptosis induced by a high concentration of calcium oxalate in the formation and development of urolithiasis. Int J Mol Med 47:289–301
Hill F, Sayer JA (2019) Precision medicine in renal stone-formers. Urolithiasis 47:99–105
Magno AL, Leatherbarrow KM, Brown SJ, Wilson SG, Walsh JP, Ward BK (2020) Functional analysis of calcium-sensing receptor variants identified in families provisionally diagnosed with familial hypocalciuric hypercalcaemia. Calcif Tissue Int 107:230–239
Wang L, Zhou Z, Yang Y, Gao P, Lin X, Wu Z (2022) A genetic polymorphism in the WDR72 gene is associated with calcium nephrolithiasis in the Chinese han population. Front Genet 13:897051
Howles SA, Wiberg A, Goldsworthy M, Bayliss AL, Gluck AK, Ng M, Grout E, Tanikawa C, Kamatani Y, Terao C et al (2019) Genetic variants of calcium and vitamin D metabolism in kidney stone disease. Nat Commun 10:5175
Peng Y, Yang C, Shi X, Li L, Dong H, Liu C, Fang Z, Wang Z, Ming S, Liu M et al (2019) Sirt3 suppresses calcium oxalate-induced renal tubular epithelial cell injury via modification of FoxO3a-mediated autophagy. Cell Death Dis 10:34
Peng Y, Fang Z, Liu M, Wang Z, Li L, Ming S, Lu C, Dong H, Zhang W, Wang Q et al (2019) Testosterone induces renal tubular epithelial cell death through the HIF-1α/BNIP3 pathway. J Transl Med 17:62
Gu Y, Shen Y, Chen W, He H, Ma Y, Mei X, Ju D, Liu H (2022) Protective effects of interleukin-22 on oxalate-induced crystalline renal injury via alleviating mitochondrial damage and inflammatory response. Appl Microbiol Biotechnol 106:2637–2649
Qiao X, Rao P, Zhang Y, Liu L, Pang M, Wang H, Hu M, Tian X, Zhang J, Zhao Y et al (2018) Redirecting TGF-β signaling through the β-catenin/foxo complex prevents kidney fibrosis. J Am Soc Nephrol 29:557–570
Yuan Q, Tang B, Zhang C (2022) Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct Target Ther 7:182
Parsana P, Amend SR, Hernandez J, Pienta KJ, Battle A (2017) Identifying global expression patterns and key regulators in epithelial to mesenchymal transition through multi-study integration. BMC Cancer 17:447
Li S, Lan Y, Wu W, Duan X, Kong Z, Wu W, Zeng G (2019) Peroxisome proliferator-activated receptor γ modulates renal crystal retention associated with high oxalate concentration by regulating tubular epithelial cellular transdifferentiation. J Cell Physiol 234:2837–2850
Tsai SH, Kinoshita M, Kusu T, Kayama H, Okumura R, Ikeda K, Shimada Y, Takeda A, Yoshikawa S, Obata-Ninomiya K et al (2015) The ectoenzyme E-NPP3 negatively regulates ATP-dependent chronic allergic responses by basophils and mast cells. Immunity 42:279–293
Letavernier E, Kauffenstein G, Huguet L, Navasiolava N, Bouderlique E, Tang E, Delaitre L, Bazin D, de Frutos M, Gay C et al (2018) ABCC6 deficiency promotes development of randall plaque. J Am Soc Nephrol 29:2337–2347
Sundararaman SS, van der Vorst EPC (2021) Calcium-sensing receptor (CaSR), its impact on inflammation and the consequences on cardiovascular health. Int J Mol Sci 22:2478
Raju P, Shashikanth N, Tsai PY, Pongkorpsakol P, Chanez-Paredes S, Steinhagen PR, Kuo WT, Singh G, Tsukita S, Turner JR (2020) Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J Clin Invest 130:5197–5208
Gamero-Estevez E, Andonian S, Jean-Claude B, Gupta I, Ryan AK (2019) Temporal effects of quercetin on tight junction barrier properties and claudin expression and localization in MDCK II cells. Int J Mol Sci 20:4889
Curry JN, Saurette M, Askari M, Pei L, Filla MB, Beggs MR, Rowe PS, Fields T, Sommer AJ, Tanikawa C et al (2020) Claudin-2 deficiency associates with hypercalciuria in mice and human kidney stone disease. J Clin Invest 130:1948–1960
Wiener SV, Chen L, Shimotake AR, Kang M, Stoller ML, Ho SP (2018) Novel insights into renal mineralization and stone formation through advanced imaging modalities. Connect Tissue Res 59:102–110
Sherer BA, Chen L, Kang M, Shimotake AR, Wiener SV, Chi T, Stoller ML, Ho SP (2018) A continuum of mineralization from human renal pyramid to stones on stems. Acta Biomater 71:72–85
Mandel N, Riese R (1991) Crystal-cell interactions: crystal binding to rat renal papillary tip collecting duct cells in culture. Am J Kidney Dis 17:402–406
Peerapen P, Thongboonkerd V (2011) Effects of calcium oxalate monohydrate crystals on expression and function of tight junction of renal tubular epithelial cells. Lab Invest 91:97–105
Hadpech S, Peerapen P, Thongboonkerd V (2022) Alpha-tubulin relocalization is involved in calcium oxalate-induced tight junction disruption in renal epithelial cells. Chem Biol Interact 368:110236
Acknowledgements
This research was funded by National Natural Science Foundation of China (No. 81970603 and No. 82100807).
Author information
Authors and Affiliations
Contributions
ZZJ, WLJ, and WZ conceived and designed the experiments; ZZJ, YYY, and GP performed the experiments; WLJ and DQ analyzed the data; ZZJ wrote the manuscript; ZTT, WZ and WLJ reviewed the manuscript; ZZJ and ZTT revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human and/or animals rights
This article does not contain any studies with animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Gao, P., Zhang, T. et al. Functional analysis reveals calcium-sensing receptor gene regulating cell–cell junction in renal tubular epithelial cells. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-03948-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-03948-3